Kinetics and Thermodynamics of Oxidation of Benzhydrol by Tetrabutyl Ammonium Bromochromatein the Presence of Oxalic Acid
S. Hemalatha1, Basim H. Asghar2 and S. Sheik Mansoor3*
1Research and Development Centre, Bharathiar University, Coimbatore – 641 046, Tamil Nadu, India.
2Department of Chemistry, Faculty of Applied Sciences, Umm Al-Qura University, P.O. Box: 9569, Makkah, Saudi Arabia.
3Department of Chemistry, C. Abdul Hakeem College (Autonomous), Melvisharam - 632 509, Tamil Nadu, India.
Corresponding Author E-mail: smansoors2000@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/320633
The kinetics of oxidation of benzhydrol (BH) by tetrabutylammonium bromochromate (TBABC) was described. Product analysis confirmed the formation of benzophenone. The reaction has been studied in the presence of oxalic acid (OA). The reaction was run under pseudo-first-order conditions. The rate of reaction is first order with respect to TBABC, BH and [H+] in the presence of oxalic acid (OA). Rates of oxidation of BH were determinedat different temperatures between 298 and 313 K andthe activation parameters were calculated. Further, a suitable mechanism has been proposed based upon the experimental results.
KEYWORDS:tetrabutylammonium bromochromate; benzhydrol; oxalic acid; thermodynamic parameters; kinetics
Download this article as:| Copy the following to cite this article: Hemalatha S, Asghar B. H, Mansoor S. S. Kinetics and Thermodynamics of Oxidation of Benzhydrol by Tetrabutyl Ammonium Bromochromatein the Presence of Oxalic Acid . Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Hemalatha S, Asghar B. H, Mansoor S. S. Kinetics and Thermodynamics of Oxidation of Benzhydrol by Tetrabutyl Ammonium Bromochromatein the Presence of Oxalic Acid . Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25262 |
Introduction
Oxidation is a key reaction for different organic synthesis. Chromium(VI) compounds have proved to be versatile reagents capable of oxidizing almost every oxidiasable functional group.A number of new chromium(VI) containing compounds, with heterocyclic bases, likeisoquinolinium bromochromate1, triphenylmethylposphonium chlorochromate2, prolinium chlorochromate3, tripropylammonium fluorochromate4, benzimidazolium fluorochromate5, quinolinium chlorochromate6, tetrahexylammonium bromochromate7, 4-benzylpyridinium fluorochromate8, tetraethyl ammonium bromochromate9and tetrabutylammonium bromochromate10have been developed in recent years to improve the selectivity of oxidation of organic compounds.
The kinetics of oxidation of benzhydrols has been studied by many reagents such as 2,2’-bipyridyl-Cu(II) permanganate11, pyridinium chlorochromate12, chloramine-T13, N-bromo succinimide14, 15, Tl(III)16 and N-bromopthalimide17. However, the kinetics of oxidation of benzhydrol by TBABC, a Cr(VI) reagent has not yetbeen studied. In continuation of our study for the oxidation of benzhydrol18,19, we studied the kinetics of oxidation of benzhydrol by TBABC in the presence of oxalic acid (OA). A probable mechanism for the oxidation is also studied.
Experimental
Materials
Tetrabutylammonium bromide and chromium trioxide were obtained from Fluka (Buchs, Switzerland). Benzhydrol (SRL,AR) andoxalic acid (Aldrich) were usedafter repeated crystallization from methanol.Acetic acid was purified by standard method and the fraction distilling at 118 oC was collected.
Preparation of Tetrabutylammonium Bromochromate, [N(C4H9)4]Cro3br
Tetrabutylammonium bromochromate (VI), [N(C4H9)4]CrO3Brwas easily prepared10 as follows: Chromium (VI) oxide (1 g, 10 mmol) was dissolved in MeCN and this solution was added to a solution of tetrabutylammonium bromide (3.22 g, 10 mmol) in MeCN under stirring at room temperature until an orange precipitate was formed. After 2 h stirring, the mixture was filtered. The solvent was evaporated at reduced pressure and the remaining solid was separated.
 |
Scheme |
Kinetic Procedure
Reactions were carried out under pseudo first-order conditionswith a known excess of [benzhydrol]o over [TBABC]o at constanttemperature using 50% acetic acid – 50% water (v/v). A decrease in [TBABC] has been followed by spectrophotometric method using UV–Vis spectrophotometer, Shimadzu UV-1800 model.
Product Analysis
Product analysis was carried under kinetic conditions. In a typical experiment, benzyhrol (1.8 g, 0.01 mol) and TBABC (8.4 g, 0.02 mol) were made up to 100 ml of the solvent (50% acetic acid – 50 % water) and kept in the dark for 24 h to ensure completion of the reaction. The solution was then treated with an excess (200 cm3) of a saturated solution of 2, 4 – dinitro phenyl hydrazine in 2 mol dm-3 HCl and kept overnight in a refrigerator. The solvent was removed and the precipitated 2, 4 – dinitro phenyl hydrazone (DNP) was filtered and recrystallised from ethanol. The identityof product was established by comparing the m.p. ofthe DNP derivative with the literature value.The m.pt of DNP was 235 – 236 oC. This value is very close to m.pt of DNP of benzophenone (lit 237 oC).
Stoichiometric Studies
The stoichiomety of the reaction was determined by carrying out several sets of experiments with varying amounts of TBABC largely in excess over benzhydrol. The estimation of unreacted TBABC showed that the following reaction
3(C6H5)2CHOH + 2Cr(VI) → 3(C6H5)2CO + 6H+ + 2Cr(III) (2)
Results and Discussion
The oxidation of benzhydrolby TBABC has been conducted in 50% acetic acid and 50% water medium at 303K, under pseudo first order conditions and the result obtained were discussed in the following paragraphs.
 |
Table 1: Rate constants for the oxidation of benzhydrolin the presence of oxalic acid by TBABC in aqueous acetic acid medium at 303 Ka Click here to View table |
Effect of varying OA concentration
The concentration of oxalic acid is varied in the range of 0.0 x 10-3 to 10.0 x 10-3 mol dm-1 at constant [TBABC], [BH] and [H+] at 303 K and the rates were measured (Table – 1). When increasing the concentration of oxalic acid, the rate linearly increases.
Effect of varying TBABC concentration in the presence of OA
The values of k1 were calculated in the presence of 6.0 x 10-3mol dm-3of oxalic acid. The concentration of TBABC was varied in the range of 0.5 x 10-3 to 2.5 x 10-3 mol dm-1 at constant [BH], [H+], [OA] at 303 K and the rates were measured (Table – 1). At various concentrations of TBABC, the k1 values remains constant. This confirms the first order dependence on TBABC.
Effect of varying benzhydrol concentration in the presence of OA
The concentration of BH is varied in the range of 1.2 x 10-2 to 3.2 x 10-2 mol dm-1 at 303 K and keeping all other reactant concentrations as constant and the rates were measured (Table – 1). The k1value linearly increases on increasing the concentration of benzhydrol. The plot of log k1 versus log [BH] gave unit slope for BH (Fig. 1). Under pseudo-first-order conditions, the plot of k1versus [BH] is linear passing through origin. These resultsconfirm the first-order nature of the reaction with respect to [BH] in the presence of OA.
Effect of varying perchloric acid concentration in the presence of OA
Perchloric acid has been used as a source of hydrogen ion in reaction medium. The concentration of H+ was varied in the range 0.12 to 0.44 mol dm-1 keeping all other reactant concentration as constant at 303 K and the rates were measured (Table – 1). The k1value linearly increases on increasing the concentration of H+. The plot of log k1versuslog [H+] is a straight line with unit slope (Fig. 2). Therefore, order with respect to H+ is one for BH in the presence of OA. TBABC may become protonated in the presence of acid and the protonated TBABC may function as an effective oxidant.
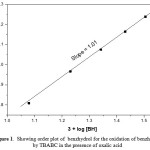 |
Figure 1: Showing order plot of benzhydrol for the oxidation of benzhydrol by TBABC in the presence of oxalic acid Click here to View figure |
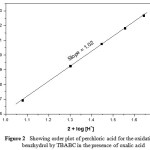 |
Figure 2: Showing order plot of perchloric acid for the oxidation of benzhydrol by TBABC in the presence of oxalic acid |
Effect of acrylonitrile and MnSO4
Oxidation of benzhydrol, under nitrogen atmosphere,failed to induce polymerization of acrylonitrile.Further, addition of acrylonitrile had no effecton the rate(Table – 1). However, the addition of Mn(II) (0.003 mol dm-3),in the form of MnSO4 retards the rate of oxidation. Hence, the presence of Cr(IV) intermediate in the oxidationof benzhydrol by Cr(VI) is confirmed20
Effect of Acidity
The reaction is catalyzed by hydrogen ions (Table 1). The acid-catalysis may well be attributed to a protonation of TBABC to give a stronger oxidant and electrophile.
O2CrBrO–N+(C4H9)4+H+ ⇌ (OH)CrBrO–N+(C4H9)4(3)
The formation of a protonated Cr (VI) species has earlier been postulated in the reactions of structurally similar PCC21 and PFC22.
Effect of solvent polarity on reaction rate in the presence of OA
The oxidation of BH has been studied in the binary mixture of acetic acid and water as the solvent medium in the presence of OA. The concentration of acetic acid was varied from 30% to 70% and the rate were measured. The reaction rate increased remarkably with the increase in the proportion of acetic acid in the solvent medium(Table – 2). Positive slope of log k1 versus 1/D plot indicates that the reaction involves a cation–dipole type of interaction in the rate determining step23 (Fig. 3).
Determination of activation parametersin the presence of OA
Rates of oxidation of benzhydrol were determinedat different temperatures between 298 and 313 K at various percentage of acetic acid-water medium in the presence of oxalic acid. Various activation parameters were calculated and the values were presented in Table -3. The entropy of activation is negative for benzhydrol in the presence of oxalic acid.
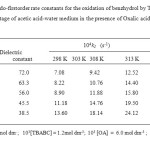 |
Table 2: Pseudo-firstorder rate constants for the oxidation of benzhydrol by TBABCat various percentage of acetic acid-water medium in the presence of Oxalic acid at various temperatures |
 |
Table 3: Second order rate constants and activation parameters for the oxidation of benzhydrol by TBABC at various percentage of acetic acid-water medium in the presence of Oxalic acid Click here to View table |
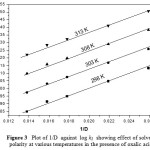 |
Figure 3: Plot of 1/D against log k1 showing effect of solvent polarity at various temperatures in the presence of oxalic acid |
Mechanism of oxidationin the presence of OA
The findings with oxalic acid can be explained by considering the reaction mechanismoutlined in Scheme – 1. Oxalic acid readily form complexes(C1) with Cr(VI) which are active oxidants24. The (C1) complex then reacts with benzhydrol to form (C2). The complex (C2) is ternary complex and it undergoes redox decomposition by two electron transfer. The rate determining step involves a cyclic transition state. In the rate determining step there is a simultaneous rupture ofC–C and C–H bonds to give a benzophenone and the Cr(IV)-OA complex.
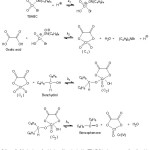 |
Scheme 1: Mechanism of oxidation of benzhydrol by TBABC in the presence of oxalic acid Click here to View Scheme |
Conclusions
The kinetics of oxidation of benzhydrol has been investigated in the presence of oxalic acid by spectrophotometric method. The values of k1 were calculated in the presence of 6.0 x 10-3mol dm-3of oxalic acid. The oxidation of benzhydrol by TBABC is first order each with respect to the benzhydrol, TBABC and hydrogen ion. The lowering of dielectric constant of reaction medium increases the reaction rate significantly. The reaction does not show the polymerization, which indicates the absence of free radical intermediate in the oxidation.
References
- Vibhute, A. Y.; Patwari, S. B.; Khansole, S. V.; Vibhute, Y. B.Chin. Chem. Lett.2009, 20, 256 – 260.
CrossRef - Hajipour, A. R.; Khazdooz, L.; Ruoho, A. E.J. Iranian Chem. Soc. 2005, 2, 315-318.
CrossRef - Mamaghani, M.; Shirini, F.; Parsa, F.Russ. J. Org. Chem.2002, 38,1113-1115.
CrossRef - Yogananth, A.; Mansoor, S.S. Oriental J. Chem. 2015, 31, 17-23.
- Malik, V. S.; Vannamuthu, I.; Shafi, S. S.; Mansoor, S. S. Oriental J. Chem.2015, 31, 77-83.
CrossRef - Elango, K. P.; Jayanthi, G.; Vijayakumar, G.J. Serb. Chem. Soc.2002, 67, 803 -808.
CrossRef - Mansoor, S.S.; Asghar, B.H. J. Indian. Chem. Soc. 2013, 90, 1395 – 1401.
- Ozgün, B.; Yaylaoglu, A.; Sendil, K. Monatsh. Chem. 2007, 138, 161 – 163.
CrossRef - Mansoor, S.S.; Shafi, S.S.Z. Phys. Chem., 2011, 225, 249 – 265.
CrossRef - Ghammamy, G.; Mehrani K.; Afrand, H.; Hajighahramani, M. Afr. J. Pure Appl. Chem.2007, 1,8-10.
- Grover, A.; Varshney, S.; Banerji, K.K. Indian J. Chem. 1996, 35B, 171 – 178.
- Venkataraman, K. S.; Sundaram, S.; Venkatasubramanian, N. Indian J. Chem. 1978, 16B, 84 – 88.
- Rangappa, K.S.; Ramachandran, H.; Mahadevappa, D.S. J. Phys. Org. Chem. 1997, 10, 159 – 166.
CrossRef - Venkatasubramanian, N.; Thiagarajan, V. Can. J. Chem.1969, 47, 694 – 697.
CrossRef - Hiran, B.L.; Malkani, R. K.; Rathore, N. Kinet. Catal. 2005, 46, 360 – 365.
CrossRef - Hiran, B. L.; Malkani, R. K.; Nalwaya, N.; Chand, K.; Rathore, N. Oxid. Commun.2004, 27, 98 – 102.
- Jagdeesh, B.; Archana, C.; Balaji, M.; Fulchand, C.; Mazahar, F.; Milind, U. J. Indian Chem. Soc. 2009, 86, 481 – 484.
- Mansoor, S. S.; Shafi, S. S.; Ahmed, S. Z. Arab. J. Chem.2013, http://dx.doi.org/10.1016/j.arabjc.2013.02.005.
CrossRef - Mansoor, S. S.; Shafi, S. S. Reac. Kinet. Mech. Cat. 2010, 100, 21 – 31.
- Karunakaran, C.; Suresh, S. J. Phys. Org. Chem.2004, 17, 88-93.
CrossRef - Sharma, V.; Sharma, P. K.; Banerji, K. K. J. Indian Chem. Soc.1997, 74, 607 – 611.
- Sharma, V.; Sharma, P.K.; Banerji, K. K.J. Chem. Research (S).1996, 290 – 291.
- Amis, E. S. Solvent Effects on Reaction Rates and Mechanisms. Academic Press, New York,1967, 42.
- Vanangamudi, G.; Srinivasan, S. E – J. Chem., 2009, 6(3),920 – 927.

This work is licensed under a Creative Commons Attribution 4.0 International License.









