Substantiation of Electron-Seeking Catalysis of Vanadocene Dichloride Oxidative Breakdown in the Process of Vanadium Backwash from Heavy Karazhanbas Oil field
Rashid I. Batkayev, Asiya F. Batkayeva, Nikolay N. Zobnin and Anastassiya Y. Kovaleva
M. Auezov South Kazakhstan State University, Kazakhstan, 160000, Shymkent, Taukekhan avenue, 5.
Corresponding Author E-mail: anastasiya2301@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/320539
In this article presents an extensive experimental results of the research of avanadocene dichloride oxidative breakdown mechanismobservable during the vanadium extraction from heavy oil. Particularly, an influence of sulfuric and nitric acid consumption on the process of demetallizationof Karazhanbas oilfield heavy oil and kinetics of this reaction were considered. It was defined that the reaction will be carried out more effective in the case of substitution of sulfuric acid as an electrophilic catalyst for immobilized Lewis acids or mineral carrierimpregnated by hydrogen acids. Immobilized catalyst use allows to exclude involvement of nitric acid as an oxidizing agentin the process of vanadocene dichloride oxidative breakdown. Also the influence of ultrasonic influencepower during the oil emulsification and electroactivated aqueous solution to the kinetics demetallization were defined.New technological scheme were proposed, which provides “oil – electroactivatedaqueous solution” emulsion preparation directly in oil-bearing strata. This engineering solution is able to reduce the number of process technological stages and to improve engineering-and-economical performance of demetallization.
KEYWORDS:Vanadium; electroactivated aqueous solution; heavy oil; oxidation-reduction potential; demetallization; productive solution
Download this article as:| Copy the following to cite this article: Batkayev R. I, Batkayeva A. F, Zobnin N. N, Kovaleva A. Y. Substantiation of Electron-Seeking Catalysis of Vanadocene Dichloride Oxidative Breakdown in the Process of Vanadium Backwash from Heavy Karazhanbas Oil field. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Batkayev R. I, Batkayeva A. F, Zobnin N. N, Kovaleva A. Y. Substantiation of Electron-Seeking Catalysis of Vanadocene Dichloride Oxidative Breakdown in the Process of Vanadium Backwash from Heavy Karazhanbas Oil field. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21500 |
Introduction
The synthesis of various organic compounds derivatives on the basis of vanadotcene is widely applied at creation of new catalytic systems for acceleration of homogeneous hydrogenation reactions of unsaturated compounds [1]. It would seem that the reverse reaction cannotlie in the sphere of researchers’. Attention to the reaction of oxidative decay vanadotsena with vanadium release in aqueous solution caused significant vanadium reserves of high-viscosity oil. The research proved, that crude oil contained about 10-12% of the vanadium in the aqueous component of the oil-in-water emulsion, i.e. in the form of soluble salts.Most of the vanadium (90%) is concentrated in the organic component of the oil-in-water emulsion.The form of vanadium in the oil particles, chemical reactions, likely in the system and mechanisms of their occurrence is very important. These parameters define the possibility and ways of vanadium extraction from crude oil, as well as an equipment used for this process and the main reactor in particular. There are many hypotheses concerning this matter. So according to the authors [2] vanadium is in the elemental state or compounds in which vanadium forms a quasi-chemical bonds easy to break. According to these data leaching metals process from the crude oil on oilfields come to transfer of vanadium and nickel containing compounds from the oil emulsion to the salt solution at the expense of their interaction with a chemical reagent.
In the acid leaching of oxidized minerals vanadium transforms into solution in the form ofvanadyl ion:
3V + 6HCl + 4HNO3 → 3VOCl2 + 4NO↑+5H2O (1)
3V + 3H2SO4 + 4HNO3 → 3VOSO4 + 4NO↑ + 5H2O (2)
On the one hand vanadyl sulfate is enough soluble in water and his formation could be explained the transfer of vanadium from the organic solution to aqueous solution. But on the other hand, according to the reference [3] the reaction (2) is possible only with concentrated sulfuric acid at the temperature 900С or by the reaction of ammonium metavanadate and ammonium phosphate in an acidic medium at the same temperature.Such conditions are not required in current methods of vanadium reextaction. Vanadium transferto the aqueous solution carries outat the temperature60-680С [4].
There is also a hypothesis that the vanadium is present in the oil as vanadylporphyrin [5].However, against this hypothesis is the fact that in the analysis of KarazhanbasandNorth Buzanchioil samples by the method of X-ray energy dispersive microanalysis revealed no adequate amounts of nitrogen. Taking into account small concentration of porphyrins, attempts were made to find the absorption spectrum in the between 1400—900 cm-1corresponds to С—О, С—N groups variations. However, such absorption bands were not found. But two absorption bands were found in the area of 1650-1600 cm-1, which indicates the presence of two conjugated C = C bonds. According to the reference [6-8] it could be an indication of vanadocene presents in the samples. Taking into account chloride background of aqueous solution, it must be assumed that the presence of the compound in the form of the dichloride.
There is also the data [5] thatthe molecular weight of the oil entails increasing the share of non-porphyrinsubstances, and the share of porphyrin complexes decreases. Karazhanbas oil has a very high molecular weight, i.e. non-porphyrin substances should be considerable amount. So, in this oil vanadium should be in some non-porphyrinform.
Material and Method
In order to research of the decay mechanism of the vanadocene dichlorideprocess, the influence of proton acids (sulfuric and nitric acids) to the main indicators of demetallization was studied. To enhance the process effect, acids were dissolved in electrical activated water (subjected to electrolytic treatment).This water has a high positive redox potential up to +850-1200 mV.An additional oxidant contributes to the process of vanadocene dichlorideoxidative decay.Oil was previously dehydrated in a centrifuge, weighed and calculated linkage fed into the reactor (5) for the demetallization (Figure 1).
 |
Figure 1: A scheme of the laboratory unit for the demetallization of oil stocks |
The water, electrochemically activated in a reactor (1) was previously supplied to the reactor in the amount of 30 ml. Together with electrochemically activated water, 50% sulfuricacid, preparedinareactor (2) and 50% nitric acid wee supplied to the main reactor. Thereactorisa container, equippedbyaheated jacket and mixer (6), driven by an electric motor (7), the rate was regulated by a laboratory autotransformer (8). The heating in the jacket was carried out by a hot water. Thewaterwasheatedina thermostat (9), thensupplied by a pump to the reactor’s jacket. The tests were carried out at temperatures of 70-80°С. The temperature in the thermostat was regulated by a contact thermometer (15). Afterthedecomposition, thepulpwas supplied for a filtration. The filtration plant consistedofa Bunsen flask (11), filter funnel (10) and vacuum pump (12). Theproductivesolutionwasdeliveredtotheanalysis, whereit was analyzed for the presence of vanadium and nickel -containing products.A unit for determination of the oxidation-reduction potential consisted of pH-meter (17) with calomel electrodes (15).
The solution after the filtration accumulated in a glass (16), where electrodes and a thermometer (18) were put in. For a uniform mixing the glass with the solution was installed on a magnetic stirrer (14). After the oxidation-reduction potential determination (17), the solution was supplied for the vanadium and nickel determination.
The investigation was carried out by a given below method. 100 g of the polymetallic raw material was placed into a reactor for the leaching, the preliminary electro-activated water in amount of 100 – 250 ml was poured there. Then, the calculated amount of sulfuric acid and NaCl was added into the prepared solution.
The pH of the obtained productive solutions was 0.25. Vanadium content in oil was determined according to State Standard 10398-76, and it amounted to 0.35%.Waste gases were subjected to analysis to determine content of sulfur oxides, nitrogen and carbon by gas-analyzer «Цвет-100».
Hydrodynamic mixing mode was maintained in all experiments at the upper boundary of turbulence with sub-critical speed.In the initial stages of research oil and electrical activated solutions mixing was performed using a laboratory shaker to obtain respective emulsions.At the final stage for the preparation of oil – activated water solution, bench-mountableultrasonic bath «УЗК» line was used with volume 1.3 l. The bath has drowned piezoceramic transducers, operating capacity of steel, resistant to acids and alkalis, two-layer heat-saving cover, drain valve, a programmable timer, system of heating and maintaining the temperature, with remote generator. To obtain the lowest possible particle emulsion, ultrasound of ultra-high frequency was used – 5MHz.
Research of the oil for the metal presence in its composition was carried out by atomic absorption spectrometry.The following devices, reagents and laboratory were used in research:ANALYST-800atomic absorption spectrophotometer, completed with options for flame atomization of samples; standard solutionof metals; TC-1 fuel; flask with 100 ml. Concentrated solution of the organic metal compounds was prepared (based on 100 ppm-1 of each metal). Working standards were prepared immediately prior to analysis by serial dilution concentrate offuel. Standard solutions were prepared with metal content: 0.02; 0.05; 0.10; 0.20; 0.50; 1.0; 2.0; 5.0 and 10.0 ppm-1. An average sample of oil (petroleum) was selected. In the 100 ml flask the sample were taken diluted (in volume) in 5, 10 or 20 times by the fuel. It is necessary to take into account the density of the fuel. If the oil sample (or oil product sample) had a poor fluidity, it was heated.
Standard solutions and solutions were analyzed spectrophotometrically at the combustion in the air mixture in the flame-acetylene or nitrogen oxide (II) – acetylene. Analytical vanadium line installed at the exit slit of the spectrophotometer.The optimal parameters, which must be followed during the tests for vanadium and nickel determination in the oil composition are shown in Table 1.
Table 1: Optimal Parameters of The Absorption Measurement
|
Indices |
Vanadium |
Nickel |
| The wavelength of the analytical line, nm |
318,4 |
341,3 |
| Current lamp with a full cathode, mA |
11 |
8 |
| The slit width, m |
160 |
40 |
| The voltage on the PMT, W |
620 |
700 |
| Scale signal amplifier |
1 |
2,5 |
| Fuel |
Acetylene |
Acetylene |
| Oxidizer |
Nitrogen oxide (II) |
Air |
| The distance from the burner to the optical axis of the device, mm |
7 |
11 |
| Acetylene pressure, psig (0,007 MPa) |
6 |
5 |
| Oxidant pressure, psig |
0,5 |
6 |
| The sample flow rate, ml / min |
6 |
6 |
| Integration time, sec |
10 |
10 |
Research of the emulsion microstructure in the oil-electroactivated solution system was carried outwith using the following equipment: scanning electron microscope JSM 6490LV (JEOL, Japan) with the system of energy dispersive microanalysis INCA Enerjy-350 (OXFORD Instruments, UK) and the system of structural analysis of polycrystalline objects HKL Basis (OXFORD Instruments, UK ). The increase was carried out from 1000 to 10000 times. At the same time the content of chemical elements from boron to uranium was analyzed. Developed method of emulsion samples preparation saving the particle size of the aqueous and organic phases in a state of initial emulsification was applied. The methodology included the work in low vacuum mode, which gave additional opportunities for the most correct evaluation of emulsion particle size.
Results and discussion
In the result of research it was defined that vanadocenehas a free electron or labile pairs in the form of unsaturated “carbon – carbon” bonds.In this respect, the double bond is the electron donor for the vanadium (which sends s-electrons to chlorine). Attack of protons proceeds on double C = C bond.In this case, the electron density of the vanadium atom moves to the hydrogen proton. Bond of cyclopentadiene ring with vanadium destroyed.Simultaneously with the proton attack the double C = C bond is attacked by O2- ion.As a result, C = C double bond breaks, stands VCl2 + ion and unprotonated molecule cyclopentenolare distinguished.
As the oxidizing agent in this reaction acts O2- ion formed in the cathode space during the electrolysis (electroactivation) or ozone water formed in the anode compartment NO3– or ions and/or SO42–, formed by electrolytic dissociation. Vanadium included in VCl2– ion in an oxidizing atmosphere is oxidized to the tetravalent state and appends hydroxyl ions from water. During this process vanadium dihydroxochloride is formedand two hydrogen protons are regenerated, which enter the process again.This mechanism is confirmed by IR spectroscopy.In the spectrogram disappears one of the two absorption bands in the 1650-1600 cm-1, indicating that the decay of one of the conjugated C = C bonds.At the same time there are characteristic absorption bands in the regions 3500-3600 cm -1 (stretching vibrations of OH- groups) and 1400-1000 cm-1 (fluctuations associated with the group C-O-H).The stretching vibrations of O-H are characteristic because they are attended by a light hydrogen atom. Since there are no conditions for the association of molecules, then the spectrogram recorded only a narrow absorption band at 3670-3580 cm-1 in cyclopentenol solution in oil (non-polar solvent).
In the absorption spectrum of the band also appeared in the area 950-1200 cm-1. From this it follows that a portion of sulfuric acid was consumed for adverse reactionsof sulfation.So sulfuric as nitric acid may act as electrophilic heterogeneous catalyst and an oxidant. To test this hypothesis was conducted a series of experiments in which acid consumption modified for demetalation process. The results are shown in Table 2.
Thermostating time in all experiments was 3 hours, pH of obtained productive solutions was 0.25. The initial concentration of vanadium in oil is 0.35%. Also, an experiment was conducted without the addition of acids using only the electro water. However, in this case no demetallation occurs, vanadium is not found in productive solutions.As a result of experiments it was proved then both acids are used in demetallization process. Formally, this on the one hand indicates that none of the acid is not a catalyst for the process, as catalyst should not be consumed in significant amounts.On the other hand the exhaust gas analysis for the content of sulfur and nitrogen oxides (SO2, NO2) showed the presence of carbon dioxide (CO2) obtained in the process of deep oxidation of petroleum hydrocarbons.
Table 2: The results of research of sulfuricandnitric acid consumption for the process of Karazhanba soil field demetallization process
|
Indicators |
Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
| Oil weight, g |
50,8 |
50,8 |
101 |
101 |
| Activated water weight, g |
30 |
30 |
30 |
30 |
| Volume of sulfuric acid 50%, ml |
10 |
5 |
10 |
10 |
| Volume of nitric acid 50%, ml |
0 |
5 |
5 |
10 |
| Concentrationofsulfuricacidfortotalvolume of emulsion, % |
7,36 |
3,97 |
4,81 |
4,81 |
| Concentrationofnitricacidfortotalvolume of emulsion, % |
0 |
3,75 |
2,38 |
4,55 |
| Concentrations | ||||
| Vanadium in oil, % |
0,35 |
0,35 |
0,35 |
0,35 |
| Vanadium in productive solution, % |
0,335 |
0,113 |
0,637 |
0,6 |
| Nickel in productive solution, % |
0,16 |
0,18 |
1,89 |
2,03 |
| Sulfur in productive solution, % |
3,12 |
1,12 |
2,96 |
1 |
| Sulfur in oil, % |
1,9 |
|||
| Terminal weight of productive solution, g |
43,95 |
43,52 |
50,50 |
57,05 |
| Initial weight of the vanadium in oil, g |
0,17 |
0,17 |
0,35 |
0,35 |
| Terminal weight of vanadium in productive solution, g |
0,14 |
0,05 |
0,32 |
0,34 |
| Recoverydegreeofvanadiumtothe productive solution, % |
83 |
28 |
91 |
97 |
| Sulfur weight in oil initial, g |
0,96 |
0,96 |
1,91 |
1,9 |
| Sulfurweightinproductivesolutionterminal, g |
1,37 |
0,48 |
1,49 |
0,57 |
| Sulfurweightinproductivesolutioninitial, g |
2,27 |
1,14 |
2,27 |
2,27 |
| Sulfurlossesfromsulfuricacidwithoil, g |
0,09 |
0,29 |
0,30 |
1,60 |
| Sulfurlossesfromsulfuricacidwithoil, % |
3,80 |
25,58 |
13,30 |
70,12 |
| Sulfurweightinoilwith a glance of income, g |
1,87 |
1,61 |
2,70 |
3,62 |
| Finite sulfurconcentrationin oil, % |
3,68 |
3,18 |
2,67 |
3,59 |
| Sulfuricacidconsumptionto 1 kgof vanadiuminproductivesolution, kg |
18,85 |
40,56 |
7,45 |
15,27 |
| Nitricacidconsumptionto 1 kgof vanadiuminproductivesolution, kg |
0 |
66,6 |
10,2 |
19,1 |
| Sulfur in gases, g |
0,82 |
0,36 |
0,48 |
0,11 |
| Nitrogen in gases, g |
0,00 |
0,73 |
0,73 |
1,46 |
| Gasvolume separated in the process, l |
0,57 |
1,42 |
1,50 |
2,41 |
| Finiteoilweighttheoretical, g |
50,49 |
50,04 |
100,20 |
99,71 |
| Oillossesintheprocessofdemetallization (theoretical), % |
0,61 |
1,49 |
0,80 |
1,28 |
Moreover, increasing the concentration of sulfur in the oil under conditions of excess nitric acid proves another side reaction –sulfation of oil hydrocarbons, what also was confirmed by IR spectral analysis of demetallized oil.
The results are given in Figure 2 and 3 in the form of graphs of the extraction degree of vanadium in the solution from a productive time and the reaction rate constant depending on the concentration of the intended catalyst – sulfuric acid on the total weight of the emulsion.
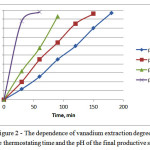 |
Figure 2: The dependence of vanadium extraction degree from thethermostatingtime and the pH of the final productive solution |
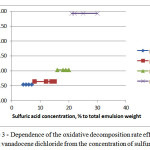 |
Figure 3: Dependence of the oxidative decomposition rate effective constant vanadocene dichloridefrom the concentration of sulfuric acid |
As can be seen from the results of the research of vanadocene dichloride oxidative decomposition kinetics, this process accelerates by specific electrophilic acid catalysis, in which there is a rapid establishment of photolytic equilibriums in solution and subsequent slow conversion by protonatedreactant into reaction products.
Taking into account that the sulfuric acid mainly acts as a catalyst and partially as an oxidant, in comparison with nitric acid, pH variation of final productive solutions made by changing the concentration of sulfuric acid on the weight of the emulsion, and the concentration of nitric acid is taken for constant.The concentration of nitric acid is taken based on the results of preliminary experiments Table 3 – 4.5% by weight of the emulsion.
The research of the electrophilic nature of catalysis of viscous oil demetalationprocess opens up great opportunities to improve this technology. Since the actual process of oil production in the field is conducted through Karajanbas fracturing oil wells out of mining as an emulsion with water to a water content of 30-40%.Prior demetalation step emulsion must be divided by separation,it is necessary to emulsify it again with water and only then fed the secondary emulsion to the reactor.This technology considerably increases the number of steps, but vanadium concentration reduced in productive solutions, operating costs are rising.
The novelty here is that we offer hydraulic fracturing to produce not by normal water, and warmed electroactivated solution. As a result it is possible to get directly the emulsion, which can be sent to the demetalation reactor after the primary separation.
Conclusions
In the result of research it was defined that demetalizationof heavyoli of Karazhanbas oilfield proceeds according to electron-seeking mechanism of oxidative reduction of vanadocene dichloride. It is recommended to carry out the process in mixed heterogeneous-heterophase mode with using immobilized catalysts. Very efficiently conduct emulsification with ultrasound at a frequency of 5 MHz at a power of 10 W/cm3. It will provide the kinetic area medium, and there is no necessity to create turbulized medium for process intensification. It was defined that emulsifying in the system oil-electro activated solution directly in oil-bearing bed is having prospects. It could allow to decrease the number of technological stages and improve the general engineering-and-economical performance of the process.
References
- Kopf-Maier P., Kopf H. Vanadocen-dichlorideinweiteresAntitumor-Agensaus der Metallocen-reihe. 1979, 34-B(6),.805-807.
- Nadirov N.K., Kotova A.V., Kamyanov V.F., New oil of Kazakhstan and their use: Metals in oil. Alma-Ata, 1984; 448
- Akhmedzhanov T.K., Nuranbayev G. and others., Patent RK 25065 dated 15.11.2011. Inventor’s certificate 2010/1293.1, publ. in 11, dated 15.12.2012, 2012.
- Batkayev R.I., NugmanovА.А., Development of complex technologies for recycling of technogenic wastes. Shymkent,2006;32
- Beysenbayev O.K., Isa A.B., Kovaleva A.Y. Research of PolyacrylonitrileSaponificationHeterophase Process Mechanism in Different Conditions// OrientJChem2015; 4-9.
- Vysotskaya N.A., Kabylbekova B. and others. Role of components containing in water on the formation corrosion – scale deposits in pipelines of heating system// Orient J Chem2015; 261-269.
- C. Lorber: “Vanadium Organometallics.” Chapter 5.01. Comprehensive Organometallic Chemistry III. Elsevier, 2007. 1-60.
CrossRef - Christoph Elschenbroich: Organometallchemie. B. G. Teubner Verlag, 2008; 50

This work is licensed under a Creative Commons Attribution 4.0 International License.









