Studies on Iron Embedded Polyesteramide Resin Derived from Melia-azedarach Seed oil-A Renewable Resource
A. Hasnat1*, M. Naseem2 and S. A. Ahmad1
1Natural Product and Polymer Research Laboratory, Department of Chemistry, G.F. College (M.J.P. Rohilkhand University), Shahajahanpur, U.P., 242001, India.
2Applied Chemistry Laboratory,University Polytechnic, Integral UniversityCampus, Shahjahanpur, U.P., 242001, India.
Corresponding Author E-mail: hasnatgfc@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/320531
Due to the depletion of petroleum oil reserves and the environmental issues both, efforts have made to utilize the renewable resources in the polymer synthesis now-a-days.Among the different renewable resources seed oils of different plants pay considerable attraction due to its unique properties. Melia azedarach is a medium sized tree largely cultivated throughout the country as a shadow tree. The seeds of the plant have approximately 40-wt% non edible oil with sufficiently high unsaturation. In the present work, oil of the Melia azedarach seeds utilized in making iron embedded polyesteramide with the objective to provide satisfactory utilization of abundantly available raw material significantly going waste in every season. The physico-chemical characterization of the polymeric material and intermediates were carried out as per standard laboratory methods.The structural elucidation of the polymeric resin was carried out by spectral analyses. The film properties of the iron embedded polyesteramide were also investigated in different environments. The results show that the iron embedded polyesteramide derived from Melia azedrach show good physic-mechanical and corrosion resistance performances in different service conditions.
KEYWORDS:Melia .azedarach seed oil; Iron-filled polyesteramide; coating material
Download this article as:| Copy the following to cite this article: Hasnat A, Naseem M, Ahmad S. A. Studies on Iron Embedded Polyesteramide Resin Derived from Melia-azedarach Seed oil-A Renewable Resource. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Hasnat A, Naseem M, Ahmad S. A. Studies on Iron Embedded Polyesteramide Resin Derived from Melia-azedarach Seed oil-A Renewable Resource. Orient J Chem 2016;32(5).Available from: http://www.orientjchem.org/?p=21933 |
Introduction
Polyesteramide resins are amide modified alkyds contain both amide and ester linkages in the polymeric chain.1-3They have reported for improved characteristics over normal alkyds in terms of hardness, ease of drying and better resistance ability towards water vapor4-6. Therefore, these resins are largely used as a coating materials or binder for paints to protect the metals from environmental corrosion and wood from biological organisms7-8. Furthermore, incorporation of metals, metalloids and organic moieties like styrene, acrylates, methacrylates in the polymeric materials improve the performances like scratch hardness9, chemical resistivity10 as well as reduces the baking temperature required for curing notably–. The primary raw material used for the synthesis of commercially important polymers has been petroleum for the last five decades11-12. However, the petroleum resources are going to deplete day by day, consequently it become costly due to scarcity. In recent years search for alternative renewable resources have been stimulated, which can be gown again and again1,2,13. Furthermore, their productivity can be raised as per demand by more plantations.
Among the different renewable resources, vegetable oils are widely used as an alternative sustainable resource for petroleum based monomers, in the synthesis of different kinds of polymers1,314,15. Numerous vegetable oils like sunflower, coconut soybean, linseed and castor have been extensively used for the syntheses low molecular weight polymers14-17. However, among these vegetable oils some are edible and other are also poses some medicinal values 17. Therefore, it is required to explore the nature’s blessings and utilized the non-edible and non-traditional seed oils in making value added polymeric materials which ultimately provide suitable utilization to them as well as reduce the pressure on traditional vegetable oils.
Melia azedarach tree yielded non edible seeds which contain about 40- wt% oil with appropriate percentage of unsaturated fatty acids18. Physico-chemical properties, especially high iodine value encourage us to utilized this vegetable oil in developing iron embedded polyesteramide with the view to provide more satisfactory utilization. In present work efforts made to utilize the seed oil of the Melia azedarach in making iron embedded polyesteramide resins with double objective utilization of non-conventional seed oil in making coating material and reduce the pressure on utilization of petrochemicals.
Experimental
Materials
Oil was extracted from air dried and crushed seeds of Melia azedarach tree (collected from the R.T.O. office compound, Shahjahanpur, India) through a soxhlet apparatus, using petroleum ether (boiling point range 60-80 0C) as a solvent. Phthalic acid, methanol, pyridine andchloroform were used of analytical grade (S.D.Fine Chemicals India). Ferric acetate (Rolex laboratory reagents, India) was used as received. Diethanolamine of analytical grade (S.D.Fine Chemicals, India) was distilled under pressure before use. Petroleum benzene,xylene (Fisher scientific, India) and were used without further purification.
Syntheses
N, N-bis(2-hydroxyethyl) Melia azedarach oil fatty amide (HEMAFA)
HMAFA was synthesized and characterized as per previously reported method19.
Polyesteramide of HEMAFA and Phthalic acid (MAPEAP)
MAPEAP was synthesized as per step-growth polymerization technique for fatty amide diol and dibasic acid15.HEMAFA and Phthalic acid in equiv-molar ratio along with 50 ml xylene as a solvent were taken in a four- necked round bottom flask attached with a Dean-stark trap, nitrogen inlet tube, thermometer and a mechanical stirrer. The Reaction mixture was heated at 180 ± 5 oC and maintained until the calculated amount of water was collected in the Dean-Stark trap. The progress of reaction was also monitored by taking acid value at regular intervals. After the completion of reaction, the reaction contents were allowed to cool at room temperature under stirring. The product was taken out from the reaction flask and excess of xylene was removed from the reaction mixture in a rotary vacuum evaporator.
Synthesis of Iron embedded Polyesteramide from MAPEAP (Fe-MAPEAP)
Iron was incorporated in MAPEAP by reacting the resin with ferric acetate (CH3COO)3Fe. The aforementioned setup was used for the synthesis of Fe-MAPEAP. MAPEAP (0.050 mole) and (CH3COO)3Fe (0.006 mole) along with 50 ml xylene were palced in afore mentioned setup. The reaction contents were heated at the rate of 10 oC/min upto 60 oC. This temperature was maintained for one hour, followed by an increase in temperature upto 160 ± 5 oC and was further maintained for 2 hours. TLC was used to monitor the progress of reaction. After the completion of reaction, the end product was allowed to cool down at room temperature and diluted in ether, then washed with 5 wt-% aqueous NaCl solution. After washing, the product was dried over anhydrous sodium sulphate and the excess of solvent was removed in a rotary vacuum evaporator under reduced pressure to obtain the Fe-MAPEAP.
Characterization
Physic-chemical characterizations like refractive index, specific gravity, acid value, iodine value of polymeric material was determine as per standard reported laboratory methods8-10. Spectral analyses of polymer sample were used for the structural elucidation. FT-IR spectrum of the polymer sample was recorded on FT-IR spectrophotometer (Perkin-Elmer) using a NaCl cell. 1H-NMR and 13C-NMR spectra were recorded on JEOL GSX 300 MHz FX-1000 spectrometer, using tetramethyl silane (TMS) as an internal reference, where as deuterated chloroform as a solvent.
Preparation of coatings
Fe-MAPEAPpolymeric resin were applied on mild steel coupons, 70x25x1 mm size for physico-mechanical test and 30x10x1 mm size for chemical/corrosion resistance test20. The mild steel strips were polished on various grade of silicon carbide papers, then washed with distilled water and finally degreased with alcohol and carbon tetrachloride. The 60-wt% solutionof Fe-MAPEAPpolymeric resin was applied on these specimens’ using brush technique20.Coated samples were baked for 5-35 minutes in an oven at different temperature (170-200 oC) to find out the optimum baking time and temperature. The best coatings were obtained by baking at 190 oC for 15 minutes. Coating thickness was measured by Elcometer (345 digital coating thickness gauge). The coating thickness were found between 120-150 µm. Flexibility (ASTMD 3281-84), scratch resistance (BS 3900) and impact resistance (IS: 101(Part5/Sec. 3) of the coatings were also tested. Corrosion tests were done in water, acid (5-wt% HCl), alkali (5-wt% NaOH) and salt (3.5-wt% NaCl) by taking them in 3 inch diameter porcelain dishes and placing the coated samples in these dishes. Periodic investigation was carried out until the coating showed evidence of softening, loss in gloss or deterioration.
Results and Discussion
Figure 1 (a,b,c) illustrate the reaction schemes for the synthesis of HEMAFA, MAPEAP and Fe-MAPEAP. The MAPEAP was synthesized by the amidation of MASO with diethanol amine and then by the step-growth polymerization of HEMAFA with phthalic acid. The resulting MAPEAP was then treated with (CH3COO)3Fe to obtain Fe-MAPEAP polymeric resin. The acid values of the reaction mixture decreases while the conversion of HEMAFA to MAPEAP and then MAPEAP to Fe-MAPEAP. This is due to the formation of repeating ester as well as iron carboxylate linkages during poly (condensation).
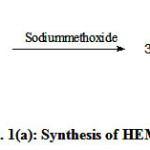 |
Figure 1(a): Synthesis of HEMAFA |
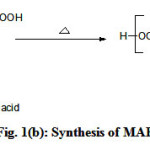 |
Figure 1(b): Synthesis of MAPEAP Click here to View Figure |
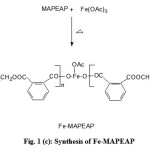 |
Figure 1(c): Synthesis of Fe-MAPEAP |
The various physico-chemical characterizations like specific gravity, refractive index, inherent viscosity, iodine value, acid value and saponification value of the developed resins were determined as per standard reported laboratory methods and summarized in Table 1.
Table 1: Physico-chemical, mechanical and chemical/corrosion resistance properties of Fe-MAPEAP polymeric resin
| Test | Fe-MAPEAP |
|
Physico-chemical analyses |
|
| Color value | 8.0 |
| Specific gravity | 0.972 |
| Refractive index | 1.509 |
| Iodine value | 30.8 |
|
Physico-mechanical properties |
|
| Bending test (1/8 in) | Passes |
| Gloss at 45o | 95 |
| Impact resistance (lb/in) | 200 |
| Scratch hardness (Kg) | 3.1 |
|
Chemical/corrosion resistance* |
|
| H2O (10 days) | E |
| HCl (3-wt%) 10 days | E |
| NaOH (2-wt%) 2 hrs | C |
| NaCl (3.5-wt%) 10 days | E |
| Xylene 10 days | D |
*A= Film detached; B= Film partially detached; C=Loss in gloss; D= Slight loss in gloss; E= Unaffected
The FT-IR spectrum of the Fe-MAPEAP shows the band of alcoholic group at 3490 cm-1 (broad band of a primary alcohol), CH2 asymmetric and symmetric stretching band at 2923 and 2856 cm-1 respectively. The bands for carbonylsof repeating ester and amide groups are observed at 1756 cm-1 and 1648cm-1respectively. The CN stretching band (C-N group) appears at 1464 cm-1. The C-O-C symmetric and asymmetric bands are observed at 1279 and 1289 cm-1.The band at 768.0 cm-1 is a characteristic of disubstituted benzene of a phthalic acid21.
The 1HNMR spectrum of Fe-MAPEAP Shows the peak at δ = 0.88-0.92 ppm for terminal methyl group. A broad peak of chain CH2 group appears at δ =1.28-1.3 ppm, protons of double bonded carbons appear at δ=5.3-5.40 ppm, where as aromatic protons are observed at δ=7.54-7.62 ppm, supporting the structure of Fe-MAPEAP as shown in Figure 1. 13 C-NMR spectrum shows the characteristic signal of carbonyl of iron corboxylate and repeating ester at δ=184.0 ppm and δ=180.2 ppm, confirm the formation of these linkages21. The additional signal such as carbonyl of amide appears at δ=176.0 ppm. Various ring carbons of benzene ring appear at δ=135.4,δ=134.2,δ=129.0 ppm. The peak for double bonded carbons of fatty amide appears at δ=129.8-130.0 ppm, different CH2 group of the fatty amide chain appear at δ=26.0-36.2 ppm, while terminal methyl group of fatty acid chain appears at 16.2 ppm.
Coating properties
Coatings of Fe-MAPEAP weredeveloped by brush technique on the required size coupons. The developed coatings were baked at different temperatures (170-220 oC) and time (10-30 mints) to obtain the optimum baking temperature and time at which coatings show best film properties. The optimum baking temperature and time for the Fe-MAPEAP polymeric resin was found 190 oC and 15 minutes.
Coatings of Fe-MAPEAP were passing the bending test on 1/8 inch conical mandrel; no visual cracks were seen, like the coating materials of other vegetable oils7,9. Coatings of Fe-MAPEAP show remarkably high values for scratch hardness and impact resistance, reasonably due to incorporation of iron, which increase the chain length of polymer, ultimately cohesive forces among the polymer chains. The presences of iron in the backbone of the polymer chain also improve the adhesion between polymeric chains and metal surface and also increase the sites for secondary bonding. The Table 1 indicates that the coatings of Fe-MAPEAP also show the good protection ability in water, acid solutions and salty environment. These are reasonably due to the high cross-linking density and adhesion of the polymeric resins towards metal surface3.
Conclusions
The synthesis of Fe-MAPEAP from Melia azedarach seed oils provides a more profitable utilization of non-traditional, non-edible and renewable resource. The synthesize resin was characterized by spectral studies as well as by physico-chemical analyses. The physico-mechanical performances and chemical/corrosion resistance abilities of Fe-MAPEAP were also investigated. The study concludes that the synthesis of Fe-MAPEAP resin provides a fruitful route for the utilisation of Melia azedarach seed oil, rot away in every season.
Acknowledgements
The authors are thankful to authorities of G.F. College for providing necessary facilities. Dr. M. Naseem is grateful to the Prof. S. W. Akhtar, Learned Vice-Chancellor, Integral University, Lucknow
References
- Ansari, S. H., Imran G., Naseem M., Ahmad, S. A. and Hasnat, A. Orient. J. Chem.2012, 28,607.
- Ansari, S. H., Naseem M., Hasnat, A. and Ahmad, S. A. Biosici. Biotech. Res. Asia,2011, 8, 829.
- Ahmad, S., Ashraf, S.M., Hasnat, A., Yadav, S. and Jamal, A. J. Appl. Polym. Sci. 2001, 82, 1856.
- Imran, G., Ahamad, S. and Ahmad S. A. Orient. J. Chem.2015, 31, 53
- Imran, G., Ahamad, S.,Altaf.I and Ahmad S. A.Chemical Science Transactions.2015, 4, (4), 1007
- Dutta, S. and Karak, N.Prog. Org. Coat.2001, 53,147.
- Zafar, F., Ashraf, S.M., Ahmad, S.Prog. Org. Coat. 2004,51, 250.
- Mahapatra, S. S. and Karak, N. Prog. Org. Coat.2004, 51, 103.
- Ahamad, S., Imran, G., Ahmad, S.A. and Hasnat, A. Orient. J. Chem.2015, 31, 1169.
- Ahmad, S., Ashraf, S.M., Hasnat, A. and Noor, A. Indian J. of Chem. Technol. 2001,8, 176.
- Ahamad, S., Ahmad, S. and Hasnat, A. Mater. Sci. Res. Ind. (In press)
- Ahmad, S., Ahmad, S. and Agnihotri S. A. Bull. Mater. Sci. 2007, 30, 31.
- Weiss, K. D. Prog. Polym. Sci.1997, 22, 203.
- Meier, M. A. R., Metzger, J.O., and Schubert, U. S. Chem. Soc. Rev. 2007, 36, 1788.
- Samarth, N. B. and Mahanwar, P. A. Open J. Org.Polym. Mater. 2015, 5, 1.
- Islam, M. R., Beg, M. D. H. and Jamari, S. S., J. Appl. Polym. Sci.2014,131, 40787.
- Olufunke, A. C. Nova J. Eng. Appl. Sci. 2014, 2, 1.
- Patel,V. C., Varughese, J., Krishnamoorthy, P. A., Jain, R. C., Singh, A. K. and Ramamoorty, M. J. Appl. Polym. Sci. 2008, 107, 1724.
- Ambasta S. P., The Useful Plants of India, CSIR, India 1994.
- Ahmad, S., Ashraf, S.M., Naqvi, F., Yadav, S. and Hasnat A. Prog. Org. Coat.2003, 47 95.
- Silverstein, R. M., Bassler, G. C.& Morril, T. C., Spectroscopic Identification of Organic Compounds, 5th edn., John Wile y & Sons, New York, 1991.

This work is licensed under a Creative Commons Attribution 4.0 International License.









