Sesmum Indicum Oil as a Potential Inhibitor for the Corrosion of Copper in Acidic Environment
Anurag Sharma1*, Anil Kumar Varshney 2 and Sarita Varshney2
1Swami Keshvanand Institute of Technology,Management and Gramothan, Jaipur,Rajasthan, India.
2Department of Chemistry, University of Rajasthan, Jaipur, Rajasthan, India.
Corresponding Author E-mail: anuragsharma20oct@yahoo.co.in
DOI : http://dx.doi.org/10.13005/ojc/320551
The inhibitory action of sesmum indicum oil on the surface of copper metal in 0.5 N HCl using gravimetric and galvanostatic polarization methods has been investigated. The rates of corrosion of copper metal with hydrochloric acid containing sesmum indicum oil were obtained as a function of the oil amount. The inhibitor efficiency was found to depend on the concentration of the sesmum indicum oil. Adsorption of sesmum indicum oil on copper metal was followed the Langmuir adsorption isotherm. The phenomenon of physical adsorption has been proposed on the basis of evaluated thermodynamic parameters.
KEYWORDS:Corrosion; Gravimetric technique; Sesmum indicum oil; Galvanostatic polarization
Download this article as:| Copy the following to cite this article: Sharma A, Varshney A. K, Varshney S. Sesmum Indicum Oil as a Potential Inhibitor for the Corrosion of Copper in Acidic Environment. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Sharma A, Varshney A. K, Varshney S. Sesmum Indicum Oil as a Potential Inhibitor for the Corrosion of Copper in Acidic Environment. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22640 |
Introduction
Control of metal’s corrosion is a sensitive part of scientific, commercially, and atmospherically point of view. The work on latest and eco friendly corrosion inhibitors has become a necessary to protect different important metals from corrosion. The appreciable works have been made to search suitable organic compounds which to be used as corrosion inhibitors in different acidic solutions, to retard the extreme attack on the metal. On account of the well known negative consequence of most artificial corrosion inhibitors and the need to work on economical, harmless and eco friendly benign processes, the efforts have been done by many investigators to attention, on the work of eco friendly corrosion inhibitor 1-8. Some of the natural oils are also found to be the good corrosion inhibitor like natural Artemisia oil 9 on steel and Jojoba oil 10 on aluminium. It has also been found that compounds which containing polar elements like nitrogen, sulphur and oxygen which have lone pairs of electrons exhibit good corrosion inhibiting property 11. The oil of Sesmum indicum is rich in manganese, copper and also contains vitamin B1 (thiamine) and vitamin E (tocopherol) .They contains lignans such as sesamol, sesamin and sesamolin. The percentage composition of fatty acid is 44%, stearic acid 4.2%, palmitic acid 9% and arachidic acid 0.7% 12. The presence of sesamol, sesamin and sesamoline in Sesmum indicum oil worked as inhibitor in 0.5N hydrochloric acid. Thermodynamic, kinetic, electrochemical and adsorption parameters for copper metal in hydrochloric acid with and without of the inhibitor were obtained and interpreted. In view of the above, it was spurred us to study the consequence of Sesmum indicum oil on copper metal’s corrosion in 0.5N HCl solution by using gravimetric method and galvanostatic polarization method.
Experimental
Gravimetric Method
Materials
Copper specimens having the composition 0.02030% Pb, 0.0297% Fe,0.0103% Ni ,0.0064% Si , 0.0087 %As, 0.0039 %Co, 0.0015 %Al, 0.0190% S and balance part Cu have been used for the experimental method.
Solutions
In the experimental, hydrochloric acid was made by analytical reagent grade chemical. Suitable strength (0.5N) of hydrochloric acid was made by de-ionized water in with and without of different concentrations of Sesmum indicum oil. The employed concentration range of sesmum indicum was of 1.0g to 6.0g in 1000ml of 0.5N hydrochloric acid.
Corrosion Rates Measurements
Gravimetric method was used to calculate the corrosion parameters. In each weight loss experiment, the test coupon’s size 3×2 ×0.2 was abraded with a different emery paper of different grades. After this, the coupons were cleaned many times with de-ionized water, after dried, coupon stored in a desiccator. Weigh the test coupon exactly, all the coupons were suspended with the help of glass hooks in 50 ml beakers which have 50 ml of 0.5 N hydrochloric acid in without and with of a certain amount of sesmum indicum. When experimental time 360 minutes was completed, the coupons were taken out, cleaned with de-ionized water, dried in oven and reweighed exactly. The difference between the weight of coupon after immersion time and initial weight of the coupons was considered the mass loss by this we can calculate the rate of corrosion given by12:
Rate of Corrosion (mill miles per year) = (87.6 W) / (dAt ) (1)
In above equation, W represents mass loss in milligrams, d represents density of the metal (gcm-3), A represents surface area of the coupon in square inch and t represents the time of experiment in hours.
The IE (%) of Sesmum indicum acting as inhibitor in 0.5 N HCl acid was obtained by the following formula:
IE (%) = (1-Wi/W0) × 100 (2)
At the room temperature, Wo and Wi are the mass loss of the copper metal without and with inhibitors amount in HCl acid. The degree of surface coverage (θ) was evaluated from formula (3)
θ = 1 – (Wi/W0) (3)
Electrochemical Polarization Method
For electrochemical polarization studies, at room temperature copper strips of similar metal coated with araldite with only open area of 1.0 cm2 was used. The electrochemical measurements were performed in a three-electrode cell. In this cell working electrode was made by copper metal which have 1cm2 open surface. Reference electrode was made by saturated calomel electrode (SCE) and auxiliary electrode was made by platinum foil.
Results and Discussion
Gravimetric Measurements
The loss of weight of copper coupons due to their immersion in solutions of 0.5 N HCl containing different concentrations of sesmum indicum was measured. It was found that the addition of sesmum indicum lowers the weight loss of the copper than its value in the free acid solution. The result indicates that the sesmum indicum oil works like effective corrosion inhibitor for copper metal in hydrochloric acid solution. The inhabitation works of sesmum indicum oil could attribute to the molecules adsorption on the copper metal’s surface. Hydrocarbon chains were oriented towards aqueous solution. These hydrocarbon chains being hydrophobic in nature repel the corrosion anions away from the metal surface thus create a hindrance between the copper metal and the corrosive territory. The efficiencies of inhibitor (IE %) with different concentrations of the Sesmum indicum oil are given in (Table 1) and shown in figure 1.
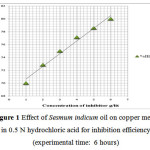 |
Figure 1: Effect of Sesmum indicum oil on copper metal in 0.5 N hydrochloric acid for inhibition efficiency (experimental time: 6 hours) |
Table 1: Inhibitor efficiency, rate of corrosion and surface cover by inhibitor at room temperature evaluated by weight loss method for copper metal in 0.5N hydrochloric acid (Experimental time: 6h)
|
Amount of Inhibitor |
Weight Loss (grams) |
Percentage Inhibitor Efficiency |
Rate of Corrosion (mill miles per year) |
Surface Cover by Inhibitor |
|
0.0 |
0.0140 |
– |
1.62 | |
|
1.0 |
0.0042 |
70.00 |
0.48 |
0.7000 |
|
2.0 |
0.0038 |
72.85 |
0.44 |
0.7285 |
|
3.0 |
0.0035 |
75.00 |
0.40 |
0.7500 |
|
4.0 |
0.0032 |
77.14 |
0.37 |
0.7714 |
|
5.0 |
0.0030 |
78.57 |
0.34 |
0.7857 |
|
6.0 |
0.0028 |
80.00 |
0.32 |
0.8000 |
Kinetics parameters of copper metal corrosion in hydrochloric acid absence and presence of Sesmum indicum oil
Figure 2, the graph between log Wf versus experimental time gave a straight line which representing that inhibition action follows first order kinetics. The data of the specific rate constant are obtained using the kinetics first order rate law13.
log (Wi -∆Wt) = – ( k /2.303) t + log Wi (4)
In above formula Wi is the starting weight of the copper metal coupon and ΔWt is the weight loss during experimental time t. The values for half-life (t1/2) were obtained by following formula14.
t ½ =0.693/k (5)
The rate constant values and half-life (t1/2) evaluated from the above formula are represented in ( Table 2).
The results represented by experiment that when amount of inhibitor increases then values of k (rate constant) decreases and half life increase.
Table 2: Kinetic data of copper with different amount of Sesmum indicum oil.
|
Concentration of inhibitor(g/lit) |
k |
t ½ |
|
0 |
6.9×10-8 |
1.0×107 |
|
1 |
1.84×10-8 |
3.7×107 |
|
2 |
1.84×10-8 |
3.7×107 |
|
3 |
1.84×10-8 |
3.7×107 |
|
4 |
1.84×10-8 |
3.7×107 |
|
5 |
1.38×10-8 |
5.0×107 |
|
6 |
9.2×10-9 |
7.5×107 |
 |
Figure 2: Kinetics graph of copper in 0.5N hydrochloric acid with absent and presence of inhibitor.
|
Thermodynamic and adsorption consideration
Experimental values evaluated for surface coverage (θ) were applied for many isotherm equations related to adsorption like Langmuir, El-Awady, Frumkin, Freundlich and Florry – Huggins. The final results represent that the isotherms that best described the adsorption feature of sesmum indicum oil on copper metal are Langmuir adsorption isotherms. Degree of surface coverage (θ) and the amount of the inhibitor in hydrochloric acid are related to following formula (6) according to Langmuir adsorption isotherm.
C/ θ = 1/Kad + C (6)
Where C is the amount of Sesmum indicum oil in hydrochloric acid, Kad is the equilibrium invariable for Sesmum indicum oil on copper surface.
A graph between C and C/θ in figure 3 produces a straight line by equation 6.
Table 3: Variable of Langmuir adsorption isotherms for the copper metal with sesmum indicum oil.
| Temperature (k) | Kad | Slope | -∆Gads | R2 |
| 298 | 3.45 | 1.2 | 29.16 | 0.999 |
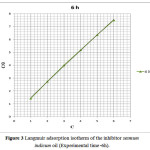 |
Figure 3: Langmuir adsorption isotherm of the inhibitor sesmum indicum oil (Experimental time-6h).
|
Galvanostatic Polarization Measurements
Galvanostatic polarization parameters were evaluated for copper metal with and without of inhibitors. Figure 4 shows the galvanostatic polarization plot for sesmum indicum in 0.5N HCl for copper metal with and without of inhibitors at optimized concentration. Galvanostatic polarization values like Ecorr (V), Icorr (A.cm-2) and percentage inhibition efficiency are given in table no. 4. Figure 5 shows that both the anodic and cathodic parts of galvanostatic polarization plot are polarized. Sesmum indicum oil adsorbed on the copper metal and hindrance the active sites and thus retards corrosion.16
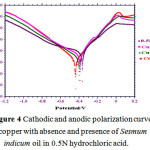 |
Figure 4: Cathodic and anodic polarization curve of copper with absence and presence of Sesmum indicum oil in 0.5N hydrochloric acid.
|
The IE% was evaluated by using formula:
IE%= 1-(I1corr / Icorr)×100 (7)
In above formula Icorr and I1corr are corrosion current densities with and without of Sesmum indicum oil in 0.5N hydrochloric acid. Ecorr values do not show any signification change suggesting sesmum indicum oil is mixed type inhibitor. The IE% evaluated from galvanostatic polarization measurements were nicely match with the gravimetric method with little changes.
Table 4: Electrochemical polarization values for copper with absence and presence of Sesmum indicum oil in 0.5N hydrochloric acid.
|
Concentration of Inhibitor |
-Ecorr(V)
|
Tafel slopes (V.dec-1) |
Icorr (A.cm-2) |
IE% |
|
|
bc |
ba |
||||
|
0.0 |
0.365 |
2.009 |
7.158 |
1.289×10-5 |
– |
|
1.0 |
0.387 |
3.908 |
9.388 |
5.950×10-6 |
53.84 |
|
3.0 |
0.399 |
5.150 |
9.081 |
3.25×10-6 |
74.78 |
|
5.0 |
0.435 |
6.616 |
9.113 |
1.714×10-6 |
86.70 |
Conclusions
Sesmum indicum oil was found to be the potential corrosion inhibitor. The efficiency of inhibitor was found 86% in 0.5 N hydrochloric acid with minor amount of sesmum indicum oil by galvanostatic method. The inhibition efficiency values obtained from gravimetric method and galvanostatic polarization measurements are found to be in close agreement. Kinetics analysis of experimental data confirmed a first order reaction. The negative value of (∆Gads) suggests that Sesmum indicum oil is effective adsorbed on copper metal and the process isspontaneous. Oil was adsorbed on the copper surface according to the Langmuir isotherms. The values of Ecorr (by the galvanostatic polarization) are almost constant which shows that the nature of Sesmum indicum oil is mixed type in 0.5 N hydrochloric acid and blocking both anodic and cathodic reaction to equal extent.
Acknowledgements
Authors are highly thankful to Head, Department of Chemistry, University of Rajasthan, Jaipur and Director (Academic), Swami Keshvanand Institute of Technology, Management & Gramothan, Jaipur for providing necessary facilities.
References
- Nnanna, Lebe, Nnanna, George, Nnakaife, Justus, Ekekwe, Nneka, Eti, Peter , Int. J. of Mat. and Chem., 2016, 6(1), 12-18.
- Al-Fakih, A.M., Aziz1, M., Sirat, H.M., J. Mater. Envcopper. Sci. 2015, 6 (5), 1480-1487.
- Okafor, P.C., Ikpi, M.I., Uwah, I.E., Ebenso, E.E., Ekpe, U.J., Umoren, S.A., Corros. Sci., 2008, 50, 2310.
- Patel,N. S., Jauhariand, S., Mehta, G. N., Al-Deyab, S. S., Warad, I., Hammouti, B., Int. J. Electrochem. Sci., , 2013, 8, 2635 – 2655.
- Odoemelam, S.A., Eddy, N.O., J. Surf. Sci. Technol., 2008, 24, 1.
- Arockia Selvi, J., Rajendran Susai, Ganga Sri, V., Amalraj, A. John, Narayanasamy, B., Port. Electrochimica Acta, 2009, 27(1), 1-11.
- Mahmoud, Magda Abdo , Mohamed, Amany , Turk J Chem, 2015, 39, 1078 – 1088.
- Fouda, A.S. , El-khateeb, A. Y., Fakih, M. , Indian J. Sci. Res. 2013, 4(2), 219-227
- Bouyanzer, A. , Hammouthi, B., Pigment & Resin Tech., 2005, 34(6), 327.
- Chetouani, A., Hammouthi,B., Benkaddour, M., Pigment & Resin Tech., 2004, 33(1), 26.
- Khaled, K.F., Abdel-Shafi, N. S., Al-Mobarak, N. A., Int. J. Electrochem. Sci., 2012, 7, 1027 – 1044.
- Pasupathy, A., Raja, T., Raja, M., Int. J. of Sci. and Res. Pub., 2015 , 5 (2), 1-3.
- M.Emran, Khadijah, Al-ahmadi, O.Arwa, Torjoman, Bayan A., Ahmed, Najla M., Sheekh, Sara N., Af. J.of pure & app.chem., 2015, 9(3),39-49.
- Atkins, P.W., Chemisorbed and Physisorbed Species, A Textbook of Physical Chemistry University Press, Oxford, 1980.
- Dasami,P.M., Parameswari, K., Chitra, S. Orient. J. Chem., 2015, 31(1), 185-191.
- Khaled, K.F., Abdelshafi, N. S., El-Maghraby, A. A., Aouniti, A., Al-Mobarak, N., Hammouti, B. Int. J. Electrochem. Sci., 2012, 7, 12706 – 12719

This work is licensed under a Creative Commons Attribution 4.0 International License.









