A Study of Fe3O4@ Si18O27 Catalyst Through Statistical-Nucleus Independent Chemical Shifts(S-NICS) Method
Neda Samiei Soofi and Majid Monajjemi
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/320504
There are no theoretical or mathematical reports of a statistical approach in NMR shielding and nucleus independent chemical shifts, while the asymmetry (η) and skew (κ) parameters are fluctuated in short distances and are alternative in long distances. In the case of axially symmetric tensor, σ22 equals either σ11 or σ33, skew is κ= ±1 and by changing asymmetry between 0 ≤ η ≤ +1 skew will be changed between -1 ≤ κ ≤ +1 , meanwhile the parameter “κ” is zero when σ22 = σiso. In this work, we have investigated a statistical method by computing of Nucleus-Independent Chemical Shifts (S-NICS) in point of probes motions in a sphere of shielding and deshielding spaces of SiO2 rings. Monajjemi in the previous work [24], has investigated a new method as the name “ S-NICS” which this method is suitable for calculation the aromaticity in the non-benzene rings such as SiO2 rings which is a famous catalyst for organic chemical synthesize and reaction. Although S-NICS values for some molecules such as benzene, borazine and naphthalene can be indicated as the aromaticity criterion, for other cases such as BnNnHx and their hydrogenated derivatives, these values indicate electromagnetic index. Finally, we have introduced a schematic diagram of statistical-nucleus independent chemical shifts for ab-initio calculations in Gaussian program, Games or other software.
KEYWORDS:naphthalene; electromagnetic index; NMR shielding
Download this article as:| Copy the following to cite this article: Soofi N. S, Monajjemi M. A Study of Fe3O4@ Si18O27 Catalyst Through Statistical-Nucleus Independent Chemical Shifts(S-NICS) Method. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Soofi N. S, Monajjemi M. A Study of Fe3O4@ Si18O27 Catalyst Through Statistical-Nucleus Independent Chemical Shifts(S-NICS) Method. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=22352 |
Introduction
Electronic towards the structural aspects have proved to be an important key in physical organic chemistry in the explanation of structures, reactivity and stabilities of various organic compounds and natural product molecules1 .
The chemical shift of a nucleus in molecular rings occurs from the nuclear shielding effect of an applied magnetic field. The magnitude of such an induced magnetic field is commensurate with the stability of the applied external magnetic field (B0). Thus the effective field (Beff) at the nucleus is given by Beff = B0 (1− σi), where “1” is the unit matrix and σi is the second-rank nuclear shielding matrix. In ordinary NMR experiments B0 is a uniform field along the z-axis and the resonance NMR frequency, νi, of a given nucleus in a molecule is therefore dependent to its gyromagnetic ratio, γi, as specified by νi = (γi/2π)B0(1 − σi)2.
The nucleus-independent chemical shift (NICS) is a computational method that calculates the absolute magnetic shielding at the center of the ring taken with reversed sign. Negative NICS values indicate aromaticity and positive values antiaromaticity3-5 .
For further investigation of aromaticity, another method called the harmonic oscillator model of aromaticity (HOMA)6 has been applied, and is distinguished as a normalized sum of the squared deviations of bond length from the normal value7.An aromatic compound has a HOMA value of one whereas a non-aromatic compound has the value 0.
Several criteria for explanation of aromaticity can be considered and may divided into five categories, which are: (1) the energetic approach to aromaticity (2) geometrical considerations (3) reactivity of aromatic compounds (4) magnetic parameters of aromaticity8-10, and the (5) Statistical-Nucleus-Independent Chemical Shifts approach (S-NICS) which is the subject of this work.
In using the energetic criterion for establishing the aromaticity of a compound, it is evident that the excess of stability of the structure is due to cyclic electron delocalization relative to suitable references systems11-12 . Moreover, Cooper, Gerratt, and Raimondi13 have developed some appropriate reference systems for calculation of the resonance energy. In using structural considerations, the geometry should show a decrease in aromaticity of bond alternation which have been reported by Julg and Kruszewski in several quantitative measurements14 . Monajjemi and Boggs have shown the low aromaticity of borazine in the rings of B18N18 and B15N15 by the non-bonded interaction method15-18 .
Regarding NMR chemical shifts and diamagnetic susceptibilities, protons attached to aromatic rings typically undergo a downfield shift from the olefin region; therefore, an up-field shift appears in the proton NMR spectrum19 . So aromaticity can be defined as the ability of a compound to sustain an induced ring current, these compounds are then called dia-tropic and antiaromatic compounds are called Para-tropic. NMR chemical shifts and diamagnetic susceptibilities , anisotropy is important when measuring a compound’s aromaticity 20.
Recently aromaticity in terms of nucleus-independent chemical shifts in long distances of NICS (1, 2.5, 3, and 3.5) Å, around the ring center, NICS (0), at the center of ring plane and aromatic ring current shielding (ARCS) were compared in several studies. In short range of distances (r<0.3) there are no theoretical or mathematical reports of statistical approach in nucleus independent chemical shift calculations, while the asymmetry (η) and skew (κ) parameters fluctuate in behavior around the center of rings.
For further discussion of statistical approach in nucleus independent chemical shift calculations, especially in short range of distances, we have focused in relaxations of CAS, dipole-dipole and contribution 21. We have shown that the asymmetry (η) and skew (κ) parameters fluctuate in behavior around the center of rings due to minimum isotropy in the center. The most fluctuations are appearing around the minimum or maximum functions mathematically.
Nuclear spin relaxation studies in the gas phase had started in 1987 22. Spin-relaxation data in the gas phase provide a stringent test of the anisotropy of an existing intermolecular potential. In some cases, spin-relaxation data is a powerful test of the anisotropic part of the intermolecular interaction. There are other observables such as the Beenakker effects, depolarized Rayleigh scattering, sound absorption, and pressure broadening of rotational lines in the IR, which are also sensitive to the anisotropy of the potential 23.
The basis of this work is on random motions of probes in the shielding and deshielding spaces of aromatic and antiaromatic molecules to consider maximum abundant of points in due to dipole–dipole, CSA and contribution relaxations. The main purpose of random displacement of various probes inside of shielding and deshielding spaces are for understanding of mechanism and consequences of anisotropic spin–spin interactions in short ranges, Although the relaxation of proton and hydrogen probes are much less, than the large ion probes such as Li+.
In CSA, relaxation Chemical shift anisotropy (CSA) originates from the orientation dependence of the chemical shift, and hence changes under rotation of the molecule and induces minor variations in the magnetic field at the site of the nucleus.
The time dependence of anisotropic interactions does however contribute to relaxation but the average amount can be time independent.
In this study, the major components of Haeberlen parameters, and chemical shift anisotropy (CSA) tensors have been calculated for borazine, benzene, naphthalene, BnNnHx rings (n=12, 15, 18) and B3N3Hn (n = 0, 2, 4, 6, 8,). The numerous random points around the center of those molecules have been produced by generation of pseudo-random numbers, which are distributed in a Gaussian function in the interval [0, 1).
The hydrogenated and dehydrogenated structures of borazine and BnNn rings have been investigated to understand more about the unknown parameters of those rings in point of electromagnetic, aromaticity, delocalization mechanism, conjugated system and hyperactive conjugation in BN alternate systems. Therefore, the hydrogenation and dehydrogenation of borazine has moved gradually in two directions toward cyclotriborazane (B3N3H12) and B3N3 respectively .
Our result has been compared by the energy decomposition analysis (EDA) method . The total bonding energy and the conjugation between three B-N bonds in borazine is significantly smaller than that for benzene and magnetically properties shows a singular behavior in borazine and B3N3Hx rings .
Fowler and Steiner computed the total current density induced by a magnetic field perpendicular to the molecular plane of borazine. They found that the currents are localized in three islands of circulation on the nitrogen atoms and concluded that borazine is moderately aromatic . Nucleus-independent chemical shift values (NICS) show a little and no evidence of ring currents, indicating with no aromaticity for borazine due to the polar B–N bond .In contrast, the S-NICS data shows a weak but stable aromaticity for borazine according to the 1999 definition provided by IUPAC definition of aromaticity.
We have optimized various isomers of B3N3 and B4N4 to understand which members of each group are more stable. Scheme 1 shows that in both groups the planar ring isomer of B3N3 and B4N4 with B and N alternate are more stable than the others.
We have discussed the electronic properties in their structures to find the reason for relative stability in these rings in point of isotropy and anisotropy. Finally the electronic structures of BnNn rings of (B3N3) N for (N= 4, 5, 6) of B12N12, B15N15 and B18N18 has been studied by S-NICS method 24.
Magnetite (Fe3O4) is the earliest discovered magnet which crystallizes in the inverse cubic spinel structure. Each cubic spinel cell contains eight interpenetrating oxygen and the tetrahedral sites, occupied by one-third of the iron atoms, form a diamond structure. The remaining Fe atoms are located at the octahedral sites with the nearest-neighbor atoms lined up as strings along six different [110] directions. In other words Fe3O4 consists of a cubic close packed array of oxide ions where all of the Fe2+ ions occupy half of the octahedral sites and the Fe3+ are split evenly across the remaining octahedral sites and the tetrahedral sites .
Both FeO and γ-Fe2O3 have a similar cubic close packed array of oxide ions and this accounts for the ready interchangeability between the three compounds on oxidation and reduction as these reactions entail a relatively small change to the overall structure therefore, Fe3O4 samples can be non-stoichiometric25 .
Fe3O4 is ferromagnetic with a curie temperature of 858 K and The ferromagnetism of Fe3O4 arises because the electron spins of the FeII and FeIII ions in the octahedral sites are coupled and the spins of the FeIII ions in the tetrahedral sites are coupled but anti-parallel to the former. Fe3O4 is used as a catalyst in the Haber process and in the water gas shift reaction .
The latter uses an HTS (high temperature shift catalyst) of iron oxide stabilized by chromium oxide . This iron-chrome catalyst is reduced at reactor start up to generate Fe3O4 from α-Fe2O3 and Cr2O3 to CrO3 . Fe3O4 is an electrical conductor with conductivity significantly higher than Fe2O3, and this is ascribed to electron exchange between the FeII and FeIII centers 25
Magnetite particles are of interests in bioscience applications such as in magnetic resonance imaging (MRI) since iron oxide magnetite nanoparticles represent a non-toxic alternative to currently employed gadolinium-based contrast agents. However, due to lack of control over the specific transformations involved in the formation of the particles, truly super paramagnetic particles have not yet been prepared from magnetite, i.e. magnetite nanoparticles that completely lose their permanent magnetic characteristic in the absence of an external magnetic field .
As a half-metallic material, Fe3O4 shows normal metallic behavior in the minority spin, while
at the same time there is a gap of~0.5 eV in the majority spin at the Fermi level. From an itinerant point of view, the high conductivity (~250 -1 cm-1) of the high-temperature phase is a natural consequence of the partially filled 3 d band of the octahedral -site Fe atoms .
Production of nano-particles can be performed chemically by taking for example mixtures of FeII and FeIII salts and mixing them with alkali to precipitate colloidal Fe3O4. The reaction conditions are critical to the process and determine the particle size .Nano particles of Fe3O4 are used as contrast agents in MRI scanning.Magnetic nanoparticles have attracted much interest not only in the field of magnetic recording but also in the areas of medical field of magnetic sensing. Especially, nanoparticles of iron oxide are reported to be applicable as a material for use in drug delivery systems, cancer therapy and MRI .
On the other hand, most of the applications require magnetic particles to disperse in a non-magnetic matrix. The matrixes play an important role in determining physical properties of the composite nanoparticle in addition to providing a means of particle dispersion.
Another important characteristic of the matrix is to act as the protection of magnetic nanoparticles against corrosion or oxidation especially in the case of metallic nanoparticles . Among carbon-based or oxide matrixes such as silica, alumina, titanic oxide or zeolite, silica can be a most suitable material for the matrix because of its non-toxicity, inertness to magnetic field and easiness to form cross-lined network structure .
Silica surfaces are chemically stable, biocompatible and can be easily functionalized for bio conjugation purpose. Hence silica-coated magnetite composite nanoparticles (Fe3O4@SiO2) have been synthesized by many groups . Recently, silica coated magnetite functionalized with γ-mercapto-propyl-trimethoxy-silane have been successfully applied to extract Cd2+, Cu2+, Hg2+, and Pb2+ from water in a wide pH range .
Catalysts play a very important role in modern science and technology as they improve reaction yields; reduce temperatures of chemical processes in synthesis.There are two main types of catalysis, heterogeneous, where the catalyst is in the solid phase with the reaction occurring on the surface and homogeneous, where the catalyst is in the same phase as the reactants .
The heterogeneous catalysts can be readily separated from the reaction mixture but the reaction rate is restricted due to their limited surface area . Meanwhile homogeneous catalysts can react very fast and provide a good conversion rate per molecule of the catalyst, but since they are miscible in the reaction medium, it can be a painstaking process to remove them from the reaction medium . The difficulty in removing homogenous catalysts from the reaction medium leads to problems in retaining the catalyst for reuse . The bridge between heterogeneous and homogeneous catalysts can be achieved through the use of Fe3O4 nanoparticles . Fe3O4 particularly is useful and important group of nanoparticles in the magnetic nanoparticles (MNPs) groups which show strong magnetic moments that are rarely retained outside of the presence of an external magnetic field. These nanoparticles may be composed of a series of materials such as metals like cobalt and nickel, alloys like iron/platinum and metal oxides like iron oxides26 .and ferrites27.Fe3O4 Nanoparticles of silica catalytic material provide the benefit of increased surface area which allows for an increased reaction rate28. Moreover, nanoparticles can permit additional catalytic functionalities due to their unique properties29.As instance, the MNPs being used to extract selected cells from biological samples and cultures30.
A few catalysis of magnetic nanostructures have been developed up to now, including the preparation of nanocomposite materials consisting of magnetic core nanoparticles which have been coated by the shell of other catalytically active nanomaterials. Another type of catalyst which is of interest for organic synthesis involves the use of organic molecules. These molecules show a large degree of specificity for their reactions and may allow a more successful reaction than conventional chemistry. Overall, the binding of catalysts to magnetic nanoparticles allows the retention of these materials after the end of the reaction for reuse.
Theoretical Background
NMR Shielding
The reduced anisotropy
![]()
and anisotropy (Δσ) with relation of
![]()
including shielding asymmetry (η) can be defined as:
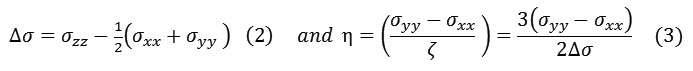
In some cases of an axially symmetric tensor,
![]()
will be zero and hence η = 0. However, the asymmetry (η) parameter indicates that how much the line figure deviates from an axially symmetric tensor, therefore, (0 ≤ η ≤ +1).
The shielding tensor can be expressed as the sum of a symmetric, an anti-symmetric, and a scalar terms, which are rank 2, rank 1 and rank zero tensors respectively as:
![]()
The total chemical shielding tensor “r” is a non-symmetric tensor that can be decomposed into three independent tensors as: (1), an isotropic component, (2) a traceless symmetric component, and (3) a traceless anti-symmetric component . In spherical tensor representation, as Haeberlen have pointed out, at a fundamental level tensors are better represented in spherical fashion, such that a general second-order property “σ” may be written as
![]()
where the number in brackets refers to tensor rank. Spherical tensors are intrinsically involved in considering the effects of tensor quantities on density matrix evolution , so the use of this representation is inevitable for such work. It is worth noting that:
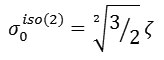
and
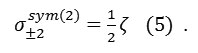
The proportionalities in these equations indicate that shielding anisotropy and asymmetry can readily be related to spherical tensor components, thus facilitating theoretical interpretation, whereas the relation between spherical tensor components and span/skew is more obscure. The isotropic tensor can be represented by a scalar average as :
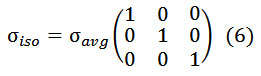
The symmetric component of the shielding tensor has tensor elements with rij = rji. This tensor is responsible for the CSA relaxation most often described in the literature and can be diagonalized by rotation into the shielding tensor principal coordinate system.The anti-symmetric tensor also induces CSA relaxation but this is almost impossible to measure because the induced effects are close to parallel to the external magnetic field which cannot be diagonalized.
By this work, in a statistical calculation we have shown that a time independent average of (Ω*) can be replaced of all above sum of asymmetric, an anti-symmetric, and a scalar terms, which are rank 2, rank 1 and rank zero tensors respectively. This method is based on random motions of probes in the shielding and deshielding spaces of aromatic and antiaromatic molecules to consider maximum abundant of relaxations points in due to dipole–dipole and spin –dipole interactions.
The magnetic environment of a spin is seldom isotropic. Therefore, is represented by a tensor of Span.
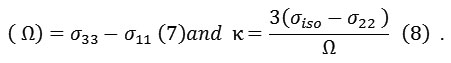
In the Herzfeld-Berger notation , tensors have explained by three parameters, which they are combination of the major components in the standard notation. Those are including, the span (Ω), which describes the maximum width of the model, (Ω ≥ 0), and the skew (κ) of the tensor which is a magnitude of the values.
The accurate formulation of the span (Ω), including the factor of (1-σref) has been described by Ω = (σ33– σ11) (1-σref) (9). In the Haeberlen-Mehring-Spiess notation, different combinations of the major components are used to explain the line figure, and is needed the major components become orderly according to their segregation from the isotropic value in this convention
The CSA relaxation rates depend on the anisotropy parameter in the standard parameters, of the shielding tensor, (σ11, σ22, σ33), are labeled according to the IUPAC rules, and they formalized and adopt the high frequency-positive order. Therefore, σ33 corresponds to the direction of minimum shielding, with the highest frequency, whenever σ11 corresponds to the direction of maximum shielding, with the lowest frequency .
Moreover the orientation of asymmetry tensoris given by
![]()
; (-1 ≤ κ ≤ +1), and related on the position of σ22 with consideration of σiso, the sign of κ is either positive or negative.
Based onour calculations especially various BnNn Rings, Benzene and naphthalene, (κ) is mostly positive31-40, and the negative values are belong to some critical or boundary points. In the case of an axially symmetric tensor, σ22 equals either σ11 or σ33 and κ= ±1 thereforea = Ω/3, and the parameter “a” and “κ” are zero when σ22 = σisoand the parameter “μ” used with the Herzfeld-Berger is related to the span of a tensor. Meanwhile, the spinning rate is given by μ = Ω*νref,
For a non-zero anti-symmetric tensor [44] give the relaxation rates
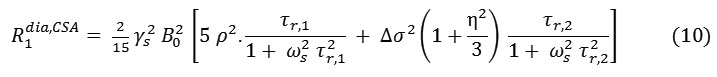
and ρ2 is defined by:
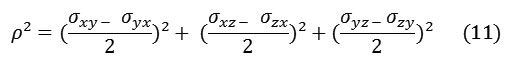
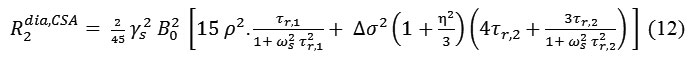
Where and correspond to the correlation times for isotropic tumbling and small-step molecular rotation, respectively and in the case of axial symmetry (η=0) or for isotropic tumbling .
Based on recent works41-66, in this study, we consider a model of SiO2 rings as a molecule for Fe3O4@SIO2 catalyst using ab initio calculations within the density functional theory (DFT) for calculating the aromaticity of rings for organic calculations.
Results and Discussion
Total shielding constants, orientations of the principal axes such as standard components,Haeberlen-Mehring, and Herzfeld-Berger parameters for Fe3O4@Si18O27 in various statistical situations have been calculated by DFT methods and the data are listed in tables1-5.
In short distances of region around the molecular centers, the asymmetric parameter (η),and the skew (κ), exhibited Gaussian distribution based on their fluctuation behavior, which is dependent on their distances from the center of the molecular rings.In contrast, of those parameters, the isotropy does not have a fluctuating behavior and it increased by increasing its distance from the center of the rings with a linear relationship. The slopes of these lines are changed, and among the levels of various distances for isolated Si18O27 and Fe3O4@Si18O27(less than 0.2 Å and more than 0.2 Å for SiO2 ring, less than 0.25 Å and more than 0.25 Å for Fe3O4@Si18O27)(Fig.1).
The isotropy during the replacing of Fe3O4@sio2 are positive which indicates negative values for aromaticity,but the slopes are decreased from the replacing from 0.1 to center (Fig.1).
As we have shown in Fig.1-7, the slopes of aromaticity curves versus distances to the center of the Fe3O4@SiO2 ring are decreased by decreasing the distances gradually which indicates distortion of aromatic electronic structures, and on the other hand these slopes are increased by increasing the distance,emphasizing a special electronic structure in Fe3O4@SiO2. Therefore, S-NICS has an increased ability to identify exact points in the area of shielding space byaromaticity criterion in such compounds via Monte Carlo stochastic calculation.
In all previous works [3,10], different basis sets yielded isotropies of various magnitude, and the criterion ofaromaticity cannot be certain by using different methods, because in multiple calculations the numerous basis sets can evaluate different isotropies for two situations of one aromatic molecule.
It is acceptable that the difference between isotropies in NICS values can express the quality of the distinct aromaticity for a few molecules, but these differences between isotropies are not able to express the mechanism of aromaticity as well as S-NICS.
In theS-NICS method via the statistical calculations, the best point of the shielding space around the center of symmetric or non-symmetric aromatic molecules can evaluated as an aromaticity criterion. and in this method the expectation of the (η*) and (κ*)have been calculated as the Gaussian curve functions versus one , two or three dimensional distances around the center of the SiO2 (Tables 1-4and Fig.1-7).
The isotropy (σiso*) which is related to all of (η*) and (κ *)and (Ω*) and ( *) is the best criterion for various aromatic molecules by the S-NICS method, which can express both qualitative and quantitative magnitudes for symmetric or non-symmetric aromatic molecules (table 3).
So “κ” can be calculated in two ways, the first one by the expectation value of the Gaussian curve (κ*) and the second one with the eqs. (27,28). Such as stochastic rules in the Monte Carlo calculation for π = 3.14 in a circle,it is evident, that the value of the Monte Carlo calculation will be more accurate by increasing the random numbers of the stochastic test, and it is significant that |κ*- κ |→0.0 by addition of random numbers in the S-NICS method.
Similar to the NICS method, in S-NICS, negative nucleus-independent chemical shifts denote aromaticity and positive values denote antiaromaticity.In S-NICS methods, the shielding and deshielding spaces are significant to discuss the mechanism of the aromatic molecules in point of ring currents, which are the circulating π electrons in an aromatic molecule produce opposite to the applied magnetics field.
The stability of the isotropy criterion is highly affectedon the best places in the shielding area spaces and it is dependent on the structures of the aromatic rings. Soby using this method, a suitable and stable magnitude of isotropy can calculated as an aromaticity criterion. It is obvious that structural factors cause changes in the magnetic field experienced by the nuclei and change the resonant frequency.Therefore the chemical shielding and many other factors such as electronegativity,hydrogen bonding, and magnetic anisotropy of π-systems will be changed because of the electrons around the proton which produce a magnetic field, countering the applied field. This reduces the field experienced at the nucleus.The electrons are said to shield the proton, an effect that is exactly dependent on the distance of the center. In addition, S-NICS can find the most accurate places for effective points for calculation of isotropy as an aromaticity criterion. The chemical shielding is a vector orientation function for all of the shielding parameters that can change in various places inside the shielding area of the rings for aromatic compounds.
The asymmetric (η),skew(k)parameters have frequent changing or fluctuatingvalues which have been modeled by a Gaussian distribution. And the shielding space around the center of benzene, naphthalene and borazine are canonical , where the (+) denotes the shielding and (–) indicates deshielding areas, and anisotropy as an orientation function has a fluctuating behavior and their values have been changed statistically in a Gaussian distribution.
On the other hand, the nearby protons will experience three fields: the applied field, the shielding field of the valence electrons and the field due to the π systems. So field lines opposed to the applied field cause a reduced field in this area equivalent to shielding, anisotropic induced magnetic field lines due to the induced circulation of the π electron in the ring area of benzene, naphthalene and borazine.S-NICS hasbeen investigated by the Monte Carlo model by computation of nucleus-independent chemical shifts in many points of shielding areas around the rings of borazine, benzene and naphthalene, by choosing specified and suitable distances (Scheme 3).The statistical simulation by the Monte Carlo method is the generation of pseudo-random numbers that are distributed in a Gaussian distribution, and the algorithm is based on a pseudorandom number generator that produces numbers x that are uniformly distributed in the interval [0, 1).
These random varietiesx are then transformed via some algorithm to create a new random variate having the required probability distribution, (Tables 1, 2). The asymmetry (η), and skew (κ) parameters fluctuate by the changing of tensors, while in the case of an axially symmetric tensor, σ22 equals either σ11 or σ33 and a = Ω/3, the span is κ= ±1 by changing asymmetry between 0 ≤ η ≤ +1.
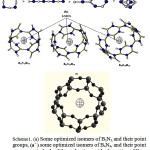 |
Scheme1: (a) Some optimized isomers of B3N3, and their point groups, (aˊ ) some optimized isomers of B4N4, and their point groups, in both of them, the rings with alternation of B and N are more stable and the arrangements of stability are shown. (b) The sphere region of S-NICS in optimized structures of B12N12, B15N15 and B18N18 Rings .all molecules are optimized with B3LYP/EPR-II level.(C)The sphere region of S-NICS in optimized structures ofSi18O27
|
Table 1: The NMR Parameters for BQ=0/09(3)
|
B3LYP/6-31G NMR=GIAO 0/09(3) |
|||||||||||
|
atom |
σ11 |
σ22 |
σ33 |
∆σ |
ƞ |
σ0iso(2) |
σ±2sym(2) |
Span(Ω) |
ƙ |
σiso |
ζzz |
|
1 |
-956.9445 |
1083.4728 |
7532.725 |
7469.4609 |
0.4097519 |
6098.5658 |
2489.8203 |
8489.6695 |
0.5193176 |
2553.0844 |
4979.6406 |
|
2 |
-3374.1985 |
848.4266 |
5337.8656 |
6600.7516 |
0.9595783 |
5389.2937 |
2200.2506 |
8712.0641 |
0.0306258 |
937.36457 |
4400.5011 |
|
3 |
178.9333 |
744.1915 |
3142.4491 |
2680.8867 |
0.3162712 |
2188.8546 |
893.6289 |
2963.5158 |
0.6185219 |
1355.1913 |
1787.2578 |
|
4 |
-1183.6157 |
-469.2794 |
6653.7304 |
7480.178 |
0.1432459 |
6107.316 |
2493.3927 |
7837.3461 |
0.8177096 |
1666.9451 |
4986.7853 |
|
5 |
110.992 |
975.0664 |
12561.8095 |
12018.78 |
0.1078405 |
9812.9335 |
4006.2601 |
12450.818 |
0.861202 |
4549.2893 |
8012.5202 |
|
6 |
-367.8772 |
16.3726 |
2243.4669 |
2419.2192 |
0.2382482 |
1975.2118 |
806.4064 |
2611.3441 |
0.7057073 |
630.6541 |
1612.8128 |
|
7 |
-2763.2793 |
159.7606 |
5052.1679 |
6353.9273 |
0.6900551 |
5187.7698 |
2117.9758 |
7815.4472 |
0.251984 |
816.2164 |
4235.9515 |
|
8 |
-6289.8512 |
187.4013 |
2580.0565 |
5631.2815 |
1.7253406 |
4597.7536 |
1877.0938 |
8869.9077 |
-0.460501 |
-1174.131 |
3754.1876 |
|
9 |
-2148.2802 |
477.4417 |
2876.8497 |
3712.269 |
1.0609638 |
3030.9439 |
1237.423 |
5025.1299 |
-0.045036 |
402.00373 |
2474.846 |
|
10 |
-6512.5485 |
7.9388 |
2252.0367 |
5504.3416 |
1.7769121 |
4494.1115 |
1834.7806 |
8764.5852 |
-0.487917 |
-1417.524 |
3669.5611 |
|
11 |
-42.9918 |
220.3325 |
4828.4089 |
4739.7386 |
0.0833351 |
3869.8385 |
1579.9129 |
4871.4007 |
0.8918897 |
1668.5832 |
3159.8257 |
|
12 |
-4147.0372 |
-106.0939 |
4209.0304 |
6335.596 |
0.9567237 |
5172.8029 |
2111.8653 |
8356.0676 |
0.0328122 |
-14.70023 |
4223.7306 |
|
13 |
117.3647 |
419.4482 |
8906.534 |
8638.1276 |
0.0524564 |
7052.7432 |
2879.3759 |
8789.1693 |
0.9312601 |
3147.7823 |
5758.7517 |
|
14 |
-794.9836 |
459.2989 |
1653.8328 |
1821.6752 |
1.0327987 |
1487.337 |
607.22505 |
2448.8164 |
-0.024399 |
439.3827 |
1214.4501 |
|
15 |
-1318.8868 |
852.2523 |
8128.2713 |
8361.5886 |
0.3894844 |
6826.9584 |
2787.1962 |
9447.1581 |
0.5403614 |
2553.8789 |
5574.3924 |
|
16 |
-4035.1798 |
216.586 |
3860.1255 |
5769.4224 |
1.1054224 |
4710.5411 |
1923.1408 |
7895.3053 |
-0.077036 |
13.8439 |
3846.2816 |
|
17 |
-3007.5149 |
557.9322 |
1705.0616 |
2929.853 |
1.8254058 |
2392.1273 |
976.61765 |
4712.5765 |
-0.513163 |
-248.1737 |
1953.2353 |
|
18 |
-2652.2661 |
200.5141 |
3365.2841 |
4591.1601 |
0.9320455 |
3748.5292 |
1530.3867 |
6017.5502 |
0.0518466 |
304.5107 |
3060.7734 |
|
19 |
-1172.0822 |
1262.6031 |
7679.6091 |
7634.3487 |
0.478368 |
6233.1912 |
2544.7829 |
8851.6913 |
0.4498938 |
2590.0433 |
5089.5658 |
|
20 |
-844.0735 |
1466.732 |
7313.6022 |
7002.273 |
0.4950119 |
5717.1225 |
2334.091 |
8157.6757 |
0.4334647 |
2645.4202 |
4668.182 |
|
21 |
-132.9882 |
742.4337 |
2027.6005 |
1722.8778 |
0.7621741 |
1406.6723 |
574.2926 |
2160.5887 |
0.189645 |
879.01533 |
1148.5852 |
|
22 |
134.332 |
752.8564 |
2813.5901 |
2369.9959 |
0.3914718 |
1935.0227 |
789.99865 |
2679.2581 |
0.5382868 |
1233.5928 |
1579.9973 |
|
23 |
-1619.9856 |
1199.4706 |
5526.3823 |
5736.6398 |
0.7372233 |
4683.7751 |
1912.2133 |
7146.3679 |
0.2109401 |
1701.9558 |
3824.4265 |
|
24 |
-347.6019 |
1091.5889 |
10841.9332 |
10469.94 |
0.206189 |
8548.3568 |
3489.9799 |
11189.535 |
0.7427613 |
3861.9734 |
6979.9598 |
|
25 |
66.743 |
1106.2361 |
10142.7306 |
9556.2411 |
0.1631645 |
7802.3523 |
3185.4137 |
10075.988 |
0.7936692 |
3771.9032 |
6370.8274 |
|
26 |
-1552.0935 |
1098.0741 |
6058.0436 |
6285.0533 |
0.6324929 |
5131.5365 |
2095.0178 |
7610.1371 |
0.3035165 |
1868.0081 |
4190.0355 |
|
27 |
-4379.5365 |
5.4372 |
3699.8494 |
5886.8991 |
1.1173048 |
4806.4569 |
1962.2997 |
8079.3859 |
-0.085472 |
-224.75 |
3924.5994 |
|
28 |
-4706.3136 |
297.386 |
6313.2225 |
8517.6863 |
0.8811723 |
6954.4069 |
2839.2288 |
11019.536 |
0.0918493 |
634.76497 |
5678.4575 |
|
29 |
-5750.8297 |
150.5728 |
5251.3484 |
8051.4769 |
1.0994385 |
6573.7624 |
2683.8256 |
11002.178 |
-0.07277 |
-116.3028 |
5367.6512 |
|
30 |
-3377.6973 |
68.9263 |
1923.1441 |
3577.5296 |
1.4451132 |
2920.9336 |
1192.5099 |
5300.8414 |
-0.300406 |
-461.8756 |
2385.0197 |
|
31 |
-2529.0503 |
415.9993 |
2861.8965 |
3918.422 |
1.1273861 |
3199.2609 |
1306.1407 |
5390.9468 |
-0.092591 |
249.61517 |
2612.2813 |
|
32 |
-13313.7769 |
236.7772 |
6478.223 |
13016.723 |
1.5615168 |
10627.72 |
4338.9076 |
19792 |
-0.369296 |
-2199.592 |
8677.8152 |
|
33 |
-342.1912 |
-59.7532 |
3910.972 |
4111.9442 |
0.1030308 |
3357.2653 |
1370.6481 |
4253.1632 |
0.8671869 |
1169.6759 |
2741.2961 |
|
34 |
105.1833 |
260.4736 |
4135.8266 |
3952.9982 |
0.0589263 |
3227.4912 |
1317.6661 |
4030.6433 |
0.9229452 |
1500.4945 |
2635.3321 |
|
35 |
-8181.1451 |
-77.0719 |
2897.3435 |
7026.452 |
1.7300495 |
5736.8638 |
2342.1507 |
11078.489 |
-0.463029 |
-1786.958 |
4684.3013 |
|
36 |
-122.1222 |
-6.8953 |
7541.5657 |
7606.0745 |
0.022724 |
6210.1063 |
2535.3582 |
7663.6879 |
0.9699291 |
2470.8494 |
5070.7163 |
|
37 |
73.7637 |
445.2708 |
8317.0012 |
8057.484 |
0.0691606 |
6578.6671 |
2685.828 |
8243.2375 |
0.9098638 |
2945.3452 |
5371.656 |
|
38 |
-2371.0975 |
638.6244 |
6844.6029 |
7710.8395 |
0.5854853 |
6295.6433 |
2570.2798 |
9215.7004 |
0.3468273 |
1704.0433 |
5140.5596 |
|
39 |
-1705.6132 |
815.6228 |
7552.004 |
7996.9992 |
0.4729091 |
6529.2833 |
2665.6664 |
9257.6172 |
0.4553164 |
2220.6712 |
5331.3328 |
|
40 |
-3476.9414 |
273.655 |
867.2673 |
2468.9105 |
2.2786952 |
2015.7831 |
822.97015 |
4344.2087 |
-0.726711 |
-778.673 |
1645.9403 |
|
41 |
-3289.9488 |
414.833 |
1998.3208 |
3435.8787 |
1.6173949 |
2805.2804 |
1145.2929 |
5288.2696 |
-0.401132 |
-292.265 |
2290.5858 |
|
42 |
-1793.8273 |
-205.034 |
2362.3919 |
3361.8226 |
0.7088982 |
2744.816 |
1120.6075 |
4156.2192 |
0.2354622 |
121.17687 |
2241.215 |
|
43 |
-4159.9595 |
141.0963 |
6540.979 |
8550.4106 |
0.754535 |
6981.1253 |
2850.1369 |
10700.939 |
0.1961348 |
840.70527 |
5700.2738 |
|
44 |
-5750.4931 |
236.0481 |
5858.1423 |
8615.3648 |
1.042302 |
7034.1581 |
2871.7883 |
11608.635 |
-0.031394 |
114.56577 |
5743.5765 |
|
45 |
-11729.9838 |
133.1489 |
9979.1707 |
15777.588 |
1.1278466 |
12881.875 |
5259.1961 |
21709.155 |
-0.092915 |
-539.2214 |
10518.392 |
|
46 |
-1.2686 |
0.3899 |
60.9549 |
61.39425 |
0.0405209 |
50.126359 |
20.46475 |
62.2235 |
0.9466922 |
20.0254 |
40.9295 |
Table 2: The NMR Parameters for BQ=0.1(1)
|
B3LYP/6-31G NMR=GIAO 0/1(1) |
|||||||||||
|
atom |
σ11 |
σ22 |
σ33 |
∆σ |
ƞ |
σ0iso(2) |
σ±2sym(2) |
Span(Ω) |
ƙ |
σiso |
ζzz |
|
1 |
-956.9445 |
1083.4728 |
7532.725 |
7469.4609 |
0.4097519 |
6098.5658 |
2489.8203 |
8489.6695 |
0.5193176 |
2553.0844 |
4979.6406 |
|
2 |
-3374.1985 |
848.4266 |
5337.8656 |
6600.7516 |
0.9595783 |
5389.2937 |
2200.2506 |
8712.0641 |
0.0306258 |
937.36457 |
4400.5011 |
|
3 |
178.9333 |
744.1915 |
3142.4491 |
2680.8867 |
0.3162712 |
2188.8546 |
893.6289 |
2963.5158 |
0.6185219 |
1355.1913 |
1787.2578 |
|
4 |
-1183.6157 |
-469.2794 |
6653.7304 |
7480.178 |
0.1432459 |
6107.316 |
2493.3927 |
7837.3461 |
0.8177096 |
1666.9451 |
4986.7853 |
|
5 |
110.992 |
975.0664 |
12561.8095 |
12018.78 |
0.1078405 |
9812.9335 |
4006.2601 |
12450.818 |
0.861202 |
4549.2893 |
8012.5202 |
|
6 |
-367.8772 |
16.3726 |
2243.4669 |
2419.2192 |
0.2382482 |
1975.2118 |
806.4064 |
2611.3441 |
0.7057073 |
630.6541 |
1612.8128 |
|
7 |
-2763.2793 |
159.7606 |
5052.1679 |
6353.9273 |
0.6900551 |
5187.7698 |
2117.9758 |
7815.4472 |
0.251984 |
816.2164 |
4235.9515 |
|
8 |
-6289.8512 |
187.4013 |
2580.0565 |
5631.2815 |
1.7253406 |
4597.7536 |
1877.0938 |
8869.9077 |
-0.460501 |
-1174.131 |
3754.1876 |
|
9 |
-2148.2802 |
477.4417 |
2876.8497 |
3712.269 |
1.0609638 |
3030.9439 |
1237.423 |
5025.1299 |
-0.045036 |
402.00373 |
2474.846 |
|
10 |
-6512.5485 |
7.9388 |
2252.0367 |
5504.3416 |
1.7769121 |
4494.1115 |
1834.7806 |
8764.5852 |
-0.487917 |
-1417.524 |
3669.5611 |
|
11 |
-42.9918 |
220.3325 |
4828.4089 |
4739.7386 |
0.0833351 |
3869.8385 |
1579.9129 |
4871.4007 |
0.8918897 |
1668.5832 |
3159.8257 |
|
12 |
-4147.0372 |
-106.0939 |
4209.0304 |
6335.596 |
0.9567237 |
5172.8029 |
2111.8653 |
8356.0676 |
0.0328122 |
-14.70023 |
4223.7306 |
|
13 |
117.3647 |
419.4482 |
8906.534 |
8638.1276 |
0.0524564 |
7052.7432 |
2879.3759 |
8789.1693 |
0.9312601 |
3147.7823 |
5758.7517 |
|
14 |
-794.9836 |
459.2989 |
1653.8328 |
1821.6752 |
1.0327987 |
1487.337 |
607.22505 |
2448.8164 |
-0.024399 |
439.3827 |
1214.4501 |
|
15 |
-1318.8868 |
852.2523 |
8128.2713 |
8361.5886 |
0.3894844 |
6826.9584 |
2787.1962 |
9447.1581 |
0.5403614 |
2553.8789 |
5574.3924 |
|
16 |
-4035.1798 |
216.586 |
3860.1255 |
5769.4224 |
1.1054224 |
4710.5411 |
1923.1408 |
7895.3053 |
-0.077036 |
13.8439 |
3846.2816 |
|
17 |
-3007.5149 |
557.9322 |
1705.0616 |
2929.853 |
1.8254058 |
2392.1273 |
976.61765 |
4712.5765 |
-0.513163 |
-248.1737 |
1953.2353 |
|
18 |
-2652.2661 |
200.5141 |
3365.2841 |
4591.1601 |
0.9320455 |
3748.5292 |
1530.3867 |
6017.5502 |
0.0518466 |
304.5107 |
3060.7734 |
|
19 |
-1172.0822 |
1262.6031 |
7679.6091 |
7634.3487 |
0.478368 |
6233.1912 |
2544.7829 |
8851.6913 |
0.4498938 |
2590.0433 |
5089.5658 |
|
20 |
-844.0735 |
1466.732 |
7313.6022 |
7002.273 |
0.4950119 |
5717.1225 |
2334.091 |
8157.6757 |
0.4334647 |
2645.4202 |
4668.182 |
|
21 |
-132.9882 |
742.4337 |
2027.6005 |
1722.8778 |
0.7621741 |
1406.6723 |
574.2926 |
2160.5887 |
0.189645 |
879.01533 |
1148.5852 |
|
22 |
134.332 |
752.8564 |
2813.5901 |
2369.9959 |
0.3914718 |
1935.0227 |
789.99865 |
2679.2581 |
0.5382868 |
1233.5928 |
1579.9973 |
|
23 |
-1619.9856 |
1199.4706 |
5526.3823 |
5736.6398 |
0.7372233 |
4683.7751 |
1912.2133 |
7146.3679 |
0.2109401 |
1701.9558 |
3824.4265 |
|
24 |
-347.6019 |
1091.5889 |
10841.9332 |
10469.94 |
0.206189 |
8548.3568 |
3489.9799 |
11189.535 |
0.7427613 |
3861.9734 |
6979.9598 |
|
25 |
66.743 |
1106.2361 |
10142.7306 |
9556.2411 |
0.1631645 |
7802.3523 |
3185.4137 |
10075.988 |
0.7936692 |
3771.9032 |
6370.8274 |
|
26 |
-1552.0935 |
1098.0741 |
6058.0436 |
6285.0533 |
0.6324929 |
5131.5365 |
2095.0178 |
7610.1371 |
0.3035165 |
1868.0081 |
4190.0355 |
|
27 |
-4379.5365 |
5.4372 |
3699.8494 |
5886.8991 |
1.1173048 |
4806.4569 |
1962.2997 |
8079.3859 |
-0.085472 |
-224.75 |
3924.5994 |
|
28 |
-4706.3136 |
297.386 |
6313.2225 |
8517.6863 |
0.8811723 |
6954.4069 |
2839.2288 |
11019.536 |
0.0918493 |
634.76497 |
5678.4575 |
|
29 |
-5750.8297 |
150.5728 |
5251.3484 |
8051.4769 |
1.0994385 |
6573.7624 |
2683.8256 |
11002.178 |
-0.07277 |
-116.3028 |
5367.6512 |
|
30 |
-3377.6973 |
68.9263 |
1923.1441 |
3577.5296 |
1.4451132 |
2920.9336 |
1192.5099 |
5300.8414 |
-0.300406 |
-461.8756 |
2385.0197 |
|
31 |
-2529.0503 |
415.9993 |
2861.8965 |
3918.422 |
1.1273861 |
3199.2609 |
1306.1407 |
5390.9468 |
-0.092591 |
249.61517 |
2612.2813 |
|
32 |
-13313.7769 |
236.7772 |
6478.223 |
13016.723 |
1.5615168 |
10627.72 |
4338.9076 |
19792 |
-0.369296 |
-2199.592 |
8677.8152 |
|
33 |
-342.1912 |
-59.7532 |
3910.972 |
4111.9442 |
0.1030308 |
3357.2653 |
1370.6481 |
4253.1632 |
0.8671869 |
1169.6759 |
2741.2961 |
|
34 |
105.1833 |
260.4736 |
4135.8266 |
3952.9982 |
0.0589263 |
3227.4912 |
1317.6661 |
4030.6433 |
0.9229452 |
1500.4945 |
2635.3321 |
|
35 |
-8181.1451 |
-77.0719 |
2897.3435 |
7026.452 |
1.7300495 |
5736.8638 |
2342.1507 |
11078.489 |
-0.463029 |
-1786.958 |
4684.3013 |
|
36 |
-122.1222 |
-6.8953 |
7541.5657 |
7606.0745 |
0.022724 |
6210.1063 |
2535.3582 |
7663.6879 |
0.9699291 |
2470.8494 |
5070.7163 |
|
37 |
73.7637 |
445.2708 |
8317.0012 |
8057.484 |
0.0691606 |
6578.6671 |
2685.828 |
8243.2375 |
0.9098638 |
2945.3452 |
5371.656 |
|
38 |
-2371.0975 |
638.6244 |
6844.6029 |
7710.8395 |
0.5854853 |
6295.6433 |
2570.2798 |
9215.7004 |
0.3468273 |
1704.0433 |
5140.5596 |
|
39 |
-1705.6132 |
815.6228 |
7552.004 |
7996.9992 |
0.4729091 |
6529.2833 |
2665.6664 |
9257.6172 |
0.4553164 |
2220.6712 |
5331.3328 |
|
40 |
-3476.9414 |
273.655 |
867.2673 |
2468.9105 |
2.2786952 |
2015.7831 |
822.97015 |
4344.2087 |
-0.726711 |
-778.673 |
1645.9403 |
|
41 |
-3289.9488 |
414.833 |
1998.3208 |
3435.8787 |
1.6173949 |
2805.2804 |
1145.2929 |
5288.2696 |
-0.401132 |
-292.265 |
2290.5858 |
|
42 |
-1793.8273 |
-205.034 |
2362.3919 |
3361.8226 |
0.7088982 |
2744.816 |
1120.6075 |
4156.2192 |
0.2354622 |
121.17687 |
2241.215 |
|
43 |
-4159.9595 |
141.0963 |
6540.979 |
8550.4106 |
0.754535 |
6981.1253 |
2850.1369 |
10700.939 |
0.1961348 |
840.70527 |
5700.2738 |
|
44 |
-5750.4931 |
236.0481 |
5858.1423 |
8615.3648 |
1.042302 |
7034.1581 |
2871.7883 |
11608.635 |
-0.031394 |
114.56577 |
5743.5765 |
|
45 |
-11729.9838 |
133.1489 |
9979.1707 |
15777.588 |
1.1278466 |
12881.875 |
5259.1961 |
21709.155 |
-0.092915 |
-539.2214 |
10518.392 |
Table 3: The NMR Parameter for BQ=0/1(2)
|
B3LYP/6-31G NMR=GIAO 0/1(2) |
|||||||||||
|
atom |
σ11 |
σ22 |
σ33 |
∆σ |
ƞ |
σ0iso(2) |
σ±sym(2) |
Span(Ω) |
ƙ |
σiso |
ζzz |
|
1 |
-956.9445 |
1083.4728 |
7532.725 |
7469.4609 |
0.4097519 |
6098.5658 |
2489.8203 |
8489.6695 |
0.5193176 |
2553.0844 |
4979.6406 |
|
2 |
-3374.1985 |
848.4266 |
5337.8656 |
6600.7516 |
0.9595783 |
5389.2937 |
2200.2506 |
8712.0641 |
0.0306258 |
937.36457 |
4400.5011 |
|
3 |
178.9333 |
744.1915 |
3142.4491 |
2680.8867 |
0.3162712 |
2188.8546 |
893.6289 |
2963.5158 |
0.6185219 |
1355.1913 |
1787.2578 |
|
4 |
-1183.6157 |
-469.2794 |
6653.7304 |
7480.178 |
0.1432459 |
6107.316 |
2493.3927 |
7837.3461 |
0.8177096 |
1666.9451 |
4986.7853 |
|
5 |
110.992 |
975.0664 |
12561.8095 |
12018.78 |
0.1078405 |
9812.9335 |
4006.2601 |
12450.818 |
0.861202 |
4549.2893 |
8012.5202 |
|
6 |
-367.8772 |
16.3726 |
2243.4669 |
2419.2192 |
0.2382482 |
1975.2118 |
806.4064 |
2611.3441 |
0.7057073 |
630.6541 |
1612.8128 |
|
7 |
-2763.2793 |
159.7606 |
5052.1679 |
6353.9273 |
0.6900551 |
5187.7698 |
2117.9758 |
7815.4472 |
0.251984 |
816.2164 |
4235.9515 |
|
8 |
-6289.8512 |
187.4013 |
2580.0565 |
5631.2815 |
1.7253406 |
4597.7536 |
1877.0938 |
8869.9077 |
-0.460501 |
-1174.131 |
3754.1876 |
|
9 |
-2148.2802 |
477.4417 |
2876.8497 |
3712.269 |
1.0609638 |
3030.9439 |
1237.423 |
5025.1299 |
-0.045036 |
402.00373 |
2474.846 |
|
10 |
-6512.5485 |
7.9388 |
2252.0367 |
5504.3416 |
1.7769121 |
4494.1115 |
1834.7806 |
8764.5852 |
-0.487917 |
-1417.524 |
3669.5611 |
|
11 |
-42.9918 |
220.3325 |
4828.4089 |
4739.7386 |
0.0833351 |
3869.8385 |
1579.9129 |
4871.4007 |
0.8918897 |
1668.5832 |
3159.8257 |
|
12 |
-4147.0372 |
-106.0939 |
4209.0304 |
6335.596 |
0.9567237 |
5172.8029 |
2111.8653 |
8356.0676 |
0.0328122 |
-14.70023 |
4223.7306 |
|
13 |
117.3647 |
419.4482 |
8906.534 |
8638.1276 |
0.0524564 |
7052.7432 |
2879.3759 |
8789.1693 |
0.9312601 |
3147.7823 |
5758.7517 |
|
14 |
-794.9836 |
459.2989 |
1653.8328 |
1821.6752 |
1.0327987 |
1487.337 |
607.22505 |
2448.8164 |
-0.024399 |
439.3827 |
1214.4501 |
|
15 |
-1318.8868 |
852.2523 |
8128.2713 |
8361.5886 |
0.3894844 |
6826.9584 |
2787.1962 |
9447.1581 |
0.5403614 |
2553.8789 |
5574.3924 |
|
16 |
-4035.1798 |
216.586 |
3860.1255 |
5769.4224 |
1.1054224 |
4710.5411 |
1923.1408 |
7895.3053 |
-0.077036 |
13.8439 |
3846.2816 |
|
17 |
-3007.5149 |
557.9322 |
1705.0616 |
2929.853 |
1.8254058 |
2392.1273 |
976.61765 |
4712.5765 |
-0.513163 |
-248.1737 |
1953.2353 |
|
18 |
-2652.2661 |
200.5141 |
3365.2841 |
4591.1601 |
0.9320455 |
3748.5292 |
1530.3867 |
6017.5502 |
0.0518466 |
304.5107 |
3060.7734 |
|
19 |
-1172.0822 |
1262.6031 |
7679.6091 |
7634.3487 |
0.478368 |
6233.1912 |
2544.7829 |
8851.6913 |
0.4498938 |
2590.0433 |
5089.5658 |
|
20 |
-844.0735 |
1466.732 |
7313.6022 |
7002.273 |
0.4950119 |
5717.1225 |
2334.091 |
8157.6757 |
0.4334647 |
2645.4202 |
4668.182 |
|
21 |
-132.9882 |
742.4337 |
2027.6005 |
1722.8778 |
0.7621741 |
1406.6723 |
574.2926 |
2160.5887 |
0.189645 |
879.01533 |
1148.5852 |
|
22 |
134.332 |
752.8564 |
2813.5901 |
2369.9959 |
0.3914718 |
1935.0227 |
789.99865 |
2679.2581 |
0.5382868 |
1233.5928 |
1579.9973 |
|
23 |
-1619.9856 |
1199.4706 |
5526.3823 |
5736.6398 |
0.7372233 |
4683.7751 |
1912.2133 |
7146.3679 |
0.2109401 |
1701.9558 |
3824.4265 |
|
24 |
-347.6019 |
1091.5889 |
10841.9332 |
10469.94 |
0.206189 |
8548.3568 |
3489.9799 |
11189.535 |
0.7427613 |
3861.9734 |
6979.9598 |
|
25 |
66.743 |
1106.2361 |
10142.7306 |
9556.2411 |
0.1631645 |
7802.3523 |
3185.4137 |
10075.988 |
0.7936692 |
3771.9032 |
6370.8274 |
|
26 |
-1552.0935 |
1098.0741 |
6058.0436 |
6285.0533 |
0.6324929 |
5131.5365 |
2095.0178 |
7610.1371 |
0.3035165 |
1868.0081 |
4190.0355 |
|
27 |
-4379.5365 |
5.4372 |
3699.8494 |
5886.8991 |
1.1173048 |
4806.4569 |
1962.2997 |
8079.3859 |
-0.085472 |
-224.75 |
3924.5994 |
|
28 |
-4706.3136 |
297.386 |
6313.2225 |
8517.6863 |
0.8811723 |
6954.4069 |
2839.2288 |
11019.536 |
0.0918493 |
634.76497 |
5678.4575 |
|
29 |
-5750.8297 |
150.5728 |
5251.3484 |
8051.4769 |
1.0994385 |
6573.7624 |
2683.8256 |
11002.178 |
-0.07277 |
-116.3028 |
5367.6512 |
|
30 |
-3377.6973 |
68.9263 |
1923.1441 |
3577.5296 |
1.4451132 |
2920.9336 |
1192.5099 |
5300.8414 |
-0.300406 |
-461.8756 |
2385.0197 |
|
31 |
-2529.0503 |
415.9993 |
2861.8965 |
3918.422 |
1.1273861 |
3199.2609 |
1306.1407 |
5390.9468 |
-0.092591 |
249.61517 |
2612.2813 |
|
32 |
-13313.7769 |
236.7772 |
6478.223 |
13016.723 |
1.5615168 |
10627.72 |
4338.9076 |
19792 |
-0.369296 |
-2199.592 |
8677.8152 |
|
33 |
-342.1912 |
-59.7532 |
3910.972 |
4111.9442 |
0.1030308 |
3357.2653 |
1370.6481 |
4253.1632 |
0.8671869 |
1169.6759 |
2741.2961 |
|
34 |
105.1833 |
260.4736 |
4135.8266 |
3952.9982 |
0.0589263 |
3227.4912 |
1317.6661 |
4030.6433 |
0.9229452 |
1500.4945 |
2635.3321 |
|
35 |
-8181.1451 |
-77.0719 |
2897.3435 |
7026.452 |
1.7300495 |
5736.8638 |
2342.1507 |
11078.489 |
-0.463029 |
-1786.958 |
4684.3013 |
|
36 |
-122.1222 |
-6.8953 |
7541.5657 |
7606.0745 |
0.022724 |
6210.1063 |
2535.3582 |
7663.6879 |
0.9699291 |
2470.8494 |
5070.7163 |
|
37 |
73.7637 |
445.2708 |
8317.0012 |
8057.484 |
0.0691606 |
6578.6671 |
2685.828 |
8243.2375 |
0.9098638 |
2945.3452 |
5371.656 |
|
38 |
-2371.0975 |
638.6244 |
6844.6029 |
7710.8395 |
0.5854853 |
6295.6433 |
2570.2798 |
9215.7004 |
0.3468273 |
1704.0433 |
5140.5596 |
|
39 |
-1705.6132 |
815.6228 |
7552.004 |
7996.9992 |
0.4729091 |
6529.2833 |
2665.6664 |
9257.6172 |
0.4553164 |
2220.6712 |
5331.3328 |
|
40 |
-3476.9414 |
273.655 |
867.2673 |
2468.9105 |
2.2786952 |
2015.7831 |
822.97015 |
4344.2087 |
-0.726711 |
-778.673 |
1645.9403 |
|
41 |
-3289.9488 |
414.833 |
1998.3208 |
3435.8787 |
1.6173949 |
2805.2804 |
1145.2929 |
5288.2696 |
-0.401132 |
-292.265 |
2290.5858 |
|
42 |
-1793.8273 |
-205.034 |
2362.3919 |
3361.8226 |
0.7088982 |
2744.816 |
1120.6075 |
4156.2192 |
0.2354622 |
121.17687 |
2241.215 |
|
43 |
-4159.9595 |
141.0963 |
6540.979 |
8550.4106 |
0.754535 |
6981.1253 |
2850.1369 |
10700.939 |
0.1961348 |
840.70527 |
5700.2738 |
|
44 |
-5750.4931 |
236.0481 |
5858.1423 |
8615.3648 |
1.042302 |
7034.1581 |
2871.7883 |
11608.635 |
-0.031394 |
114.56577 |
5743.5765 |
|
45 |
-11729.9838 |
133.1489 |
9979.1707 |
15777.588 |
1.1278466 |
12881.875 |
5259.1961 |
21709.155 |
-0.092915 |
-539.2214 |
10518.392 |
|
46 |
-1.4025 |
0.303 |
60.591 |
61.14075 |
0.041842 |
49.919384 |
20.38025 |
61.9935 |
0.9449781 |
19.8305 |
40.7605 |
Table 4: The NMR Parameter for BQ=0/1(3)
|
B3LYP/6-31G NMR=GIAO 0/1(3) |
|||||||||||
|
|
σ11 |
σ22 |
σ33 |
∆σ |
ƞ |
σ0iso(2) |
σ±sym(2) |
Span(Ω) |
ƙ |
σiso |
ζzz |
|
1 |
-956.9445 |
1083.4728 |
7532.725 |
7469.4609 |
0.4097519 |
6098.5658 |
2489.8203 |
8489.6695 |
0.5193176 |
2553.0844 |
4979.6406 |
|
2 |
-3374.1985 |
848.4266 |
5337.8656 |
6600.7516 |
0.9595783 |
5389.2937 |
2200.2506 |
8712.0641 |
0.0306258 |
937.36457 |
4400.5011 |
|
3 |
178.9333 |
744.1915 |
3142.4491 |
2680.8867 |
0.3162712 |
2188.8546 |
893.6289 |
2963.5158 |
0.6185219 |
1355.1913 |
1787.2578 |
|
4 |
-1183.6157 |
-469.2794 |
6653.7304 |
7480.178 |
0.1432459 |
6107.316 |
2493.3927 |
7837.3461 |
0.8177096 |
1666.9451 |
4986.7853 |
|
5 |
110.992 |
975.0664 |
12561.8095 |
12018.78 |
0.1078405 |
9812.9335 |
4006.2601 |
12450.818 |
0.861202 |
4549.2893 |
8012.5202 |
|
6 |
-367.8772 |
16.3726 |
2243.4669 |
2419.2192 |
0.2382482 |
1975.2118 |
806.4064 |
2611.3441 |
0.7057073 |
630.6541 |
1612.8128 |
|
7 |
-2763.2793 |
159.7606 |
5052.1679 |
6353.9273 |
0.6900551 |
5187.7698 |
2117.9758 |
7815.4472 |
0.251984 |
816.2164 |
4235.9515 |
|
8 |
-6289.8512 |
187.4013 |
2580.0565 |
5631.2815 |
1.7253406 |
4597.7536 |
1877.0938 |
8869.9077 |
-0.460501 |
-1174.131 |
3754.1876 |
|
9 |
-2148.2802 |
477.4417 |
2876.8497 |
3712.269 |
1.0609638 |
3030.9439 |
1237.423 |
5025.1299 |
-0.045036 |
402.00373 |
2474.846 |
|
10 |
-6512.5485 |
7.9388 |
2252.0367 |
5504.3416 |
1.7769121 |
4494.1115 |
1834.7806 |
8764.5852 |
-0.487917 |
-1417.524 |
3669.5611 |
|
11 |
-42.9918 |
220.3325 |
4828.4089 |
4739.7386 |
0.0833351 |
3869.8385 |
1579.9129 |
4871.4007 |
0.8918897 |
1668.5832 |
3159.8257 |
|
12 |
-4147.0372 |
-106.0939 |
4209.0304 |
6335.596 |
0.9567237 |
5172.8029 |
2111.8653 |
8356.0676 |
0.0328122 |
-14.70023 |
4223.7306 |
|
13 |
117.3647 |
419.4482 |
8906.534 |
8638.1276 |
0.0524564 |
7052.7432 |
2879.3759 |
8789.1693 |
0.9312601 |
3147.7823 |
5758.7517 |
|
14 |
-794.9836 |
459.2989 |
1653.8328 |
1821.6752 |
1.0327987 |
1487.337 |
607.22505 |
2448.8164 |
-0.024399 |
439.3827 |
1214.4501 |
|
15 |
-1318.8868 |
852.2523 |
8128.2713 |
8361.5886 |
0.3894844 |
6826.9584 |
2787.1962 |
9447.1581 |
0.5403614 |
2553.8789 |
5574.3924 |
|
16 |
-4035.1798 |
216.586 |
3860.1255 |
5769.4224 |
1.1054224 |
4710.5411 |
1923.1408 |
7895.3053 |
-0.077036 |
13.8439 |
3846.2816 |
|
17 |
-3007.5149 |
557.9322 |
1705.0616 |
2929.853 |
1.8254058 |
2392.1273 |
976.61765 |
4712.5765 |
-0.513163 |
-248.1737 |
1953.2353 |
|
18 |
-2652.2661 |
200.5141 |
3365.2841 |
4591.1601 |
0.9320455 |
3748.5292 |
1530.3867 |
6017.5502 |
0.0518466 |
304.5107 |
3060.7734 |
|
19 |
-1172.0822 |
1262.6031 |
7679.6091 |
7634.3487 |
0.478368 |
6233.1912 |
2544.7829 |
8851.6913 |
0.4498938 |
2590.0433 |
5089.5658 |
|
20 |
-844.0735 |
1466.732 |
7313.6022 |
7002.273 |
0.4950119 |
5717.1225 |
2334.091 |
8157.6757 |
0.4334647 |
2645.4202 |
4668.182 |
|
21 |
-132.9882 |
742.4337 |
2027.6005 |
1722.8778 |
0.7621741 |
1406.6723 |
574.2926 |
2160.5887 |
0.189645 |
879.01533 |
1148.5852 |
|
22 |
134.332 |
752.8564 |
2813.5901 |
2369.9959 |
0.3914718 |
1935.0227 |
789.99865 |
2679.2581 |
0.5382868 |
1233.5928 |
1579.9973 |
|
23 |
-1619.9856 |
1199.4706 |
5526.3823 |
5736.6398 |
0.7372233 |
4683.7751 |
1912.2133 |
7146.3679 |
0.2109401 |
1701.9558 |
3824.4265 |
|
24 |
-347.6019 |
1091.5889 |
10841.9332 |
10469.94 |
0.206189 |
8548.3568 |
3489.9799 |
11189.535 |
0.7427613 |
3861.9734 |
6979.9598 |
|
25 |
66.743 |
1106.2361 |
10142.7306 |
9556.2411 |
0.1631645 |
7802.3523 |
3185.4137 |
10075.988 |
0.7936692 |
3771.9032 |
6370.8274 |
|
26 |
-1552.0935 |
1098.0741 |
6058.0436 |
6285.0533 |
0.6324929 |
5131.5365 |
2095.0178 |
7610.1371 |
0.3035165 |
1868.0081 |
4190.0355 |
|
27 |
-4379.5365 |
5.4372 |
3699.8494 |
5886.8991 |
1.1173048 |
4806.4569 |
1962.2997 |
8079.3859 |
-0.085472 |
-224.75 |
3924.5994 |
|
28 |
-4706.3136 |
297.386 |
6313.2225 |
8517.6863 |
0.8811723 |
6954.4069 |
2839.2288 |
11019.536 |
0.0918493 |
634.76497 |
5678.4575 |
|
29 |
-5750.8297 |
150.5728 |
5251.3484 |
8051.4769 |
1.0994385 |
6573.7624 |
2683.8256 |
11002.178 |
-0.07277 |
-116.3028 |
5367.6512 |
|
30 |
-3377.6973 |
68.9263 |
1923.1441 |
3577.5296 |
1.4451132 |
2920.9336 |
1192.5099 |
5300.8414 |
-0.300406 |
-461.8756 |
2385.0197 |
|
31 |
-2529.0503 |
415.9993 |
2861.8965 |
3918.422 |
1.1273861 |
3199.2609 |
1306.1407 |
5390.9468 |
-0.092591 |
249.61517 |
2612.2813 |
|
32 |
-13313.7769 |
236.7772 |
6478.223 |
13016.723 |
1.5615168 |
10627.72 |
4338.9076 |
19792 |
-0.369296 |
-2199.592 |
8677.8152 |
|
33 |
-342.1912 |
-59.7532 |
3910.972 |
4111.9442 |
0.1030308 |
3357.2653 |
1370.6481 |
4253.1632 |
0.8671869 |
1169.6759 |
2741.2961 |
|
34 |
105.1833 |
260.4736 |
4135.8266 |
3952.9982 |
0.0589263 |
3227.4912 |
1317.6661 |
4030.6433 |
0.9229452 |
1500.4945 |
2635.3321 |
|
35 |
-8181.1451 |
-77.0719 |
2897.3435 |
7026.452 |
1.7300495 |
5736.8638 |
2342.1507 |
11078.489 |
-0.463029 |
-1786.958 |
4684.3013 |
|
36 |
-122.1222 |
-6.8953 |
7541.5657 |
7606.0745 |
0.022724 |
6210.1063 |
2535.3582 |
7663.6879 |
0.9699291 |
2470.8494 |
5070.7163 |
|
37 |
73.7637 |
445.2708 |
8317.0012 |
8057.484 |
0.0691606 |
6578.6671 |
2685.828 |
8243.2375 |
0.9098638 |
2945.3452 |
5371.656 |
|
38 |
-2371.0975 |
638.6244 |
6844.6029 |
7710.8395 |
0.5854853 |
6295.6433 |
2570.2798 |
9215.7004 |
0.3468273 |
1704.0433 |
5140.5596 |
|
39 |
-1705.6132 |
815.6228 |
7552.004 |
7996.9992 |
0.4729091 |
6529.2833 |
2665.6664 |
9257.6172 |
0.4553164 |
2220.6712 |
5331.3328 |
|
40 |
-3476.9414 |
273.655 |
867.2673 |
2468.9105 |
2.2786952 |
2015.7831 |
822.97015 |
4344.2087 |
-0.726711 |
-778.673 |
1645.9403 |
|
41 |
-3289.9488 |
414.833 |
1998.3208 |
3435.8787 |
1.6173949 |
2805.2804 |
1145.2929 |
5288.2696 |
-0.401132 |
-292.265 |
2290.5858 |
|
42 |
-1793.8273 |
-205.034 |
2362.3919 |
3361.8226 |
0.7088982 |
2744.816 |
1120.6075 |
4156.2192 |
0.2354622 |
121.17687 |
2241.215 |
|
43 |
-4159.9595 |
141.0963 |
6540.979 |
8550.4106 |
0.754535 |
6981.1253 |
2850.1369 |
10700.939 |
0.1961348 |
840.70527 |
5700.2738 |
|
44 |
-5750.4931 |
236.0481 |
5858.1423 |
8615.3648 |
1.042302 |
7034.1581 |
2871.7883 |
11608.635 |
-0.031394 |
114.56577 |
5743.5765 |
|
45 |
-11729.9838 |
133.1489 |
9979.1707 |
15777.588 |
1.1278466 |
12881.875 |
5259.1961 |
21709.155 |
-0.092915 |
-539.2214 |
10518.392 |
|
46 |
-1.2697 |
0.3822 |
60.9302 |
61.37395 |
0.040373 |
50.109825 |
20.458 |
62.1999 |
0.9468826 |
20.014233 |
40.916 |
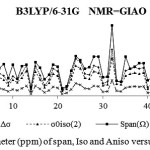 |
Figure 1: NMR Parameter (ppm) of span, Iso and Aniso versus atomic number |
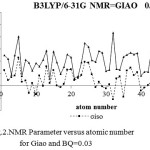 |
Figure 2: NMR Parameter versus atomic number for Giao and BQ=0.03 |
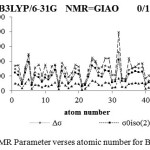 |
Figure 3: NMR Parameter verses atomic number for BQ=0.1 |
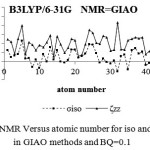 |
Figure 4: NMR Versus atomic number for iso and ZZ in GIAO methods and BQ=0.1 |
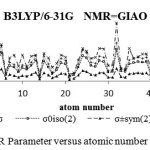 |
Figure 5: NMR Parameter versus atomic number for Span |
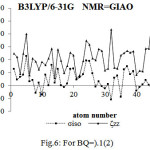 |
Figure 6: For BQ=).1(2) |
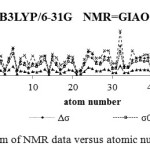 |
Figure 7: ppm of NMR data versus atomic number |
 |
Figure 8: ZigmaIso for the Giao=0.1(3) Click here to View Figure |
References
- Minkin, V. J. ;Glukhovtsev, M. N. ;Simkin, B.Y. ;Electronic and Structural Aspects, Wiley, New York, 1984.
- Mason, J. ;Solid State Nucl.Magn.Reson.1993,2, 285.
- Schleyer, P. v. R. ; Jiao, H. ; van EikemaHommes, N. J. R. ;Malkin, V. G. ;Malkina, O. L. ; J. Am. Chem. Soc. 1997, 119, 12669.
- Schleyer, P. v. R. ;Maerker, C. ;Dransfeld, A. ; Jiao, H. ; van EikemaHommes, N. J. R. J. Am. Chem.Soc.1996,118, 6317.
- Schleyer, P. v. R. ; Jiao, H. ;Pure. Appl. Chem.1996, 68, 209.
- Kruszewski, J .; Krygowski, T. M. ;Tetrahedron Letters,1972,36, 3839.
- Stepien, B.T. ;Krygowski, T.M. ;Cyranski, M.K. ;Mlochowski, J.;Orioli, P. ;Abbate, F. ARKIVOC,2004, 3,185.
- Katritzky, A. R. ; Barczynski, P.;Musumarra, G. ; Pisano, D. ;Szafran, M. J. Am. Chem.Soc.1989, 111, 7.
- Feixas, F. ;Matito, E. ;Poater, J. ;Solà, M. Journal of Computational Chemistry.2008, 29, 543.
- Katritzky, A. R.;Karelson, M.;Sild, S.;Krygowski, T. M.; Jug. K. J. Org. Chem.1998,63, 5228.
- Fias, S.; Van Damme, S.; Bultinck,P.; Journal of Computational Chemistry. 2008,29, 358.
- Hehre, W. J. R.;Ditchfield, Radom, L.;Pople, J. A. J. Am. Chem. Soc.1970, 92, 4796.
- Cooper, D. L.;Gerratt, J.; Raimondi, M. Nature,1986,323, 699.
- Julg, A. Francois, Ph.;Theor. Chim.Acta.1967,7, 249.
- Monajjemi, M.;Struct. Chem.2012,23, 551.
- Monajjemi, M.; Lee, V. S.;Khaleghian, M.;Honarparvar, B.;Mollaamin. F.;J. Phys. Chem. C.2010114, 15315.
- Monajjemi, M.;Khaleghian, M.;J. Cluster Sci.2011, 22, 673.
- Monajjemi, M.; Boggs, J.E, J. Phys. Chem. A,20133.
- Frueh, D.;Nucl.P.;Reson.M,Spectrosc.2002,41, 305.
- Jiao, H.; .Schleyer, P. v. R,J. Am. Chem. Soc. 1995,117, 11529.
- Martin, N. H.; Nance, K.H.;Journal of Molecular Graphics and Modelling.2002,21, 51.
- Luginbhl, P.;Wuthrich , K.;Progress in Nuclear Magnetic Resonance Spectroscopy.2002, 40, 199.
- Magn. R. L.;Reson. Rev.1987,12, 91.
- Monajjemi,M.; Mohammadian,T.N.; J. Comput. Theor.Nanosci.2015, 12, 4895-4914.
- Greenwood, Norman N.; Earnshaw, Alan Chemistry of the Elements (2nd ed.). Butterworth-Heinemann.(1997). ISBN 0080379419.
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Chem. Rev. 2008, 108, 2064–2110.
- Kodama, R.H. Magnetic nanoparticles. J. Magn. Magn.Mater.1999, 200, 359–372.
- Chang, L.L.; Erathodiyil, N.; Ying, J.Y. Acc. Chem. Res. 2012, 46, 1825–1837.
- Fujishima, A.; Zhang, X.; Tryk, D.A. Surf. Sci. Rep. 2008, 63, 515–582.
- Wang, X.; Starz-Gaiano, M.; Bridges, T.; Montell, D. Protoc, Exch,2008, 28.
- Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010,41(2-3), 142-149
- Ali R. Ilkhani .; MajidMonajjemi, Computational and Theoretical Chemistry.2015, 1074 19–25
- Monajjemi,M *.; Bagheri,S.; Moosavi,M.S..; Moradiyeh,N.; Zakeri,M.; Attarikhasraghi,N.; Saghayimarouf,N.; Niyatzadeh,G.; Shekarkhand,M.; Mohammad S. Khalilimofrad, Ahmadin,H.; Ahadi,M.; Molecules 2015, 20, 21636–21657;
- Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM, 2005, 714 (1), 28, 43-60.
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S..; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009,83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Monajjemi, M.; JafariAzan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.201152(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M. TheorChemAcc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;.Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Monajjemi, M. Biophysical Chemistry.2015207,114 –127
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids.2011, 49 (5), 561-571
- Amiri, A.; Babaeie, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2008, 46, 4, 379-389
- Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015,54, 8, 08510
- Naghsh,F, orient.jchem,2015, 31(1) 465-478
- Chitsazan, A, orient.jchem,2015, 31(1) 393- 408
- Barmaki, Z, orient.jchem, 2015, 31(3) 1723-1733
- Bonsakhteh, B.; Rustaiyan, A.H, orient.jchem, 2014, 30(4) 1703-1718

This work is licensed under a Creative Commons Attribution 4.0 International License.









