A Novel method in Biosynthesis Silver Nanocomposite
Ferdos Parsa Mehr*, Houman Jafarpourgolroudbary, Behrooz Katir, Mohammad Reza Tizchang
Department of Chemical Engineering, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: f.parsa.en.p.ch@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320538
In this work,Polyacrylaimde grafted carboxymethyl starch (PAAm-g-CMS) with different nitrogen contents was prepared using ultra violet /photoinitiator (UV/PI) system where the water soluble 4-(trimethylammoniummethyl) benzophenone chloride was used as the photoinitiator. PAAm-g-CMS was further utilized to prepare PAAm-g- CMS silver nanoparticles (PAAm-g-CMS-AgNPs) composite. The latter was 12prepared using silver nitrate as a precursor and PAAm-g-CMS as both reducing and capping agent under alkaline conditions. The as prepared PAAm-g-CMS-AgNPs composite was characterized by FTIR and by measuring the absorbance of its colloidal solution using UV-vis spectrophotometer. The size and shape of the nanoparticles were measured by TEM. Reproducibility of the synthesis method was also tested. Highest absorbance of the colloidal solution of PAAm-g-CMS-AgNPs was obtained when PAAm-g-CMS (Nitrogen content (%) =7.7) concentration of 0.8% (w/v); AgNO3 concentration of 900 ppm; pH 12; temperature 80C for 3h were used. 20 TEM showed round shape nanoparticles with size varies from 1-11 nm.
KEYWORDS:Photo-initiator; Carboxymethyl Starch; Acrylamide; PAAm-g-CMS-AgNPs composite
Download this article as:| Copy the following to cite this article: Mehr F. P, Jafarpourgolroudbary H, Katir B, Tizchang M. R. A Novel Photo-Grafting of Acrylamide Onto Carboxymethyl Starch. 2. Utilization of 1 CMS-G-Paam in Synthesis of CMS-G-Paam-Agnpsnano Composite. Orient J Chem 2016;32(5). |
| Copy the following to cite this URL: Mehr F. P, Jafarpourgolroudbary H, Katir B, Tizchang M. R. A Novel Photo-Grafting of Acrylamide Onto Carboxymethyl Starch. 2. Utilization of 1 CMS-G-Paam in Synthesis of CMS-G-Paam-Agnpsnano Composite. Orient J Chem 2016;32(5). Available from: http://www.orientjchem.org/?p=21515 |
Introduction
It is understood that silver displays compelling antibacterial properties with low toxicity for humans and animals when compared with other heavy metals and some organic antibacterial agents. Silver and silver compounds are compelling for both Grams – ve and Gram +ve bacteria, though the proficiency of traditional antibiotics changes with the species of bacteria [1]. Utilizing AgNPs promptsexpanding the number of particles per unit area and, thus, antibacterial impacts can be maximized [2]. Among the perceived novel methodologies, hydrogel or macroscopic gels 52 have been utilized as promising formats or nanopots to prepare nanoparticles that brought an idea for more up to date composite/hybrid materials [3,4]. The accessible free-network spaces between hydrogel networks reserve to grew and stabilize the nanoparticles. In detail, in-situ reduction of metal ions and stabilization of particles can be clarified as (a) first metal ions are anchored by functional groups of hydrogel networks and significant measures of metal ions are captured in the free-network spaces of hydrogels, (b) then diminishment procedure happens where decreasing specialists utilized for this reason, (c) the framed particles are all around balanced out by the hydrogel system chains [5,6,7,8]. The formed nanoparticles in their network effectively inhibit the aggregation for longer periods and can be extracted into water whenever they are required for usage.
Because of their good bio-compatibility over biological molecules, tissues, cells, etc, nanocomposite systems are very suitable for bio-medical applications. Lee & Huang prepared a series of antibacterial superabsorbents containing silver hydrogel nanocomposites by inverse suspension polymerization [9,10].
Materials and methods
CMS (DS= 0.3) was prepared according to a reported method [11, 12]. In this method, Native maize starch was soaked in ethanol and aqueous solution of sodium hydroxide for 30 min. After soaking, sodium salt solution of monochloroaceticacid was added dropwise. The whole components were stirred for three hours at 70C. The produced CMS was filtered and washed with 80% ethanol then oven dried at 70C.
Definite weight of PAAm-g-CMS was dissolved in distilled water using heating magnetic stirrer. After complete dissolution, the desired pH (11-12.5) of the solution was adjusted using dilute solution of sodium hydroxide. The temperature was raised gradually to reach to the desired temperature (50–80C). Certain volume ofdiluted silver nitrate solution was then added dropwise to the mixture keeping in mind that the total volume of the reaction mixture is 100 cm3 and silver nitrate concentration varies from 100-1000 ppm. The reaction mixture was kept under continuous stirring for different durations (15–12 min). After addition of silver nitrate solution, the reaction medium starts to acquire a clear yellow color which develops to brown indicating the formation of silver nanoparticles (Ag0). After completion of the synthesis, the colloidal solution formed was checked for the presence of excess silver ions (Ag+) using dilutesNaCl solution. When no white precipitate is observed, this means the complete transformation of (Ag+) to silver nanoparticles (Ag0). The development of the reaction was evaluated by withdrawing aliquots from resultant Ag0 colloidal solution at given time intervals (15, 30, 60, 90 and 120 min). The prepared AgNPs were assessed by measuring the absorbance of the colloidal solution using UV spectrophotometer. Sample showed highest absorbance (at the optimum condition of preparation) was subjected to TEM for evaluating the shape and size of theAgNPs obtained.
Results and discussions
Four PAAm-g-CMS samples with different nitrogen contents: 9.2%, 7.7%, 7.2% and 5.8% were prepared by controlling the reaction conditions. The photopolymerization mechanism of the preparation of PAAm-g-CMS is mentioned in details in a previous work [13,14]. However, it would be benefited to remention it in this section for sake of understanding the discussions of some results obtained.
In addition to the original reducing aldehydic end groups, original OH groups, C=O, and COOH groups and CONH2, new reducing groups are generated as a result of the oxidative degradation of PAAm-g-CMS molecule during the synthesis of silver nanoparticles under the effect of sodium hydroxide (pH 11-12.5) at relatively high temperature (50–80∘C). In addition, new COOH and NH2 groups are generated as a result of the hydrolysis of the CONH2 groups due to the presence of sodium hydroxide at high temperature in the synthesis medium. While the reduction keeps on, silver nanoparticles grow gradually and PAAm-g-CMS will further form a stable protection layer on the theAgNPs surface. Stabilization of AgNPs takes place byelectrostatic interaction between Ag+ cations and functional groups originally present or generated, as a result of the reaction conditions, on the PAAm-g-CMS polymer.
Factors affecting the reduction and stability of AgNPs are given in what follows. The shape and size of the formed silver nanoparticles along with the reproducibility of the method used are also discussed.
As pH is a very important factor in the synthesis of PAAm-g-CMS AgNPs, a range of alkaline pHs was applied to the preparation medium. Fig. 1 shows the UV–vis spectra of the colloidal solution of AgNPs obtained when PAAm-g-CMS was used as reducing and stabilizing agent at different pHs (11-12.5), different durations (15-120 min) while keeping the rest of reaction conditions constant. The kinetics of the reaction was performed by picking samples after different lengths of time. Fig. 1 focuses on the effect of pH on the formation of silver nanoparticles at 120 min.
Results Fig. 1reveal a number of observations which may be summarized as follows: (a) Increasing the pH of the solution is accompanied by appreciable changes in the electronic absorption spectra; (b) A band at about 405 nm starts to appear and reaches its maximum intensity at pH 12 and 12.5. (c) Regardless of the pH, all spectra showed a peak at 405 nm, (d) The peak at 405 nm is weak, broad with low absorbance at the initial stages of reaction (15-30 min) and (d) at all pHs the band becomes sharper, stronger and symmetrical, with a pronounced bell shape at 60-120 min.(Fig. 1)
TEM images of silver sol are shown in Fig. 2. These observations indicate the adsorption and/or deposition of silver nanoparticles onto the surface of roughly sphere-shaped polydispersed. It can be noticed that the graphs show that the particles are spherical in shape.
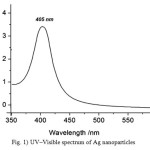 |
Figure 1: UV–Visible spectrum of Ag nano particles
|
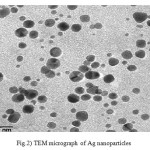 |
Figure 2: TEM micrograph of Ag nano particles
|
Conclusions
Poly acrylaimdecarboxymethyl starch graft copolymers (PAAm-g-CMS) with different nitrogen contents were prepared using Ultra Violet/photoinitiator (UV/PI) system where the water soluble 4-(trimethylammoniummethyl)benzophenone chloride was used as the photoinitiator. The poly acrylamide-g-carboxymethyl starch silver anoparticles (PAAm-g-CMS-AgNPs) composite was prepared using silver nitrate as a precursor and PAAm-g-CMS as a reducing and capping agent under alkaline solution. Parameters affecting the synthesis of silver NPs such as silver nitrate and PAAm-g-CMS concentrations as well as reaction time, reaction temperature, N% of PAAm-g-CMS and pH are studied. The synthesized PAAm-g-CMS-AgNPswere assessed by measuring the absorbance of the colloidal solution using UV spectrophotometer. The size and the shape were measured by TEM. Reproducibility of the synthesis method was also tested. The participation of PAAm-g-CMS in the synthesis of AgNPs was confirmed by FTIR spectr. Results revealed that optimum conditions for obtaining PAAm-g-CMS-AgNPs are: PAAm-g-CMS (N% 7.7) concentration, 0.8% (w/v); AgNO3 concentration, 900 ppm; pH 12; temp., 80oC for 3h. TEM showed round shape nanoparticles with size varies from 1-11 nm. The PAAm-g-CMS-AgNPs. Hydrogel powder was prepared from PAAm-g-CMS-AgNPs colloidal solution. The produced PAAm-g-CMS-AgNPs hydrogel showed swelling capacity% of 697.
References
- Silvert, P. Y.; Urbina, R. H.; Elhsissen,K. T. J. Mater. Chem., 1997, 7, 293.
CrossRef - Egorova, E. M.; Revina,A. A. Colloids Surf. A: Physicochem. Eng. Asp.,2006,168, 87.
CrossRef - Xie, Y.; R. Ye; Liu, H. Colloids Surf. A: Physicochem. Eng. Asp.,2006, 279, 175.
CrossRef - Shrivastava. S.; Bera T.; Roy A.; Singh G.; Ramachandrarao Dash P.. Journal of Nanotechnology, 200718., 225103-225112.
- Shrivastava.S.;Bera T.; Roy A.; Singh G.; Ramachandrarao Dash P.. Journal of Nanotechnology, 200718., 225103-225112.
- Roh Y, Bai J, Lauf RJ, Mcmillan AD, Phelps TJ, Rawn CJ, Solid State Commun, 2001,118, 529-34.
CrossRef - Sastry M.; Ahmad A.; Islam Khan M.; Kumar R. Current Science, 2003, 85,162-70.
- Ankamwar B.; Damle C.; Ahmad A.; and Sastry M., Journal of NanoscienceNanotechnology, 2005,5, . 1665-71
CrossRef - Priya Banerjee.; MantoshSatapathy.; AniruddhaMukhopahayay and PapitaDas .Bioresources and Bioprocessing, 2014, 1, 2- 10.
- Bansal V.; Rautaray D.; Ahmad D. and Sastry M. Journal of Material Chemistry 2004.,14. 3303 –3305.
CrossRef - GardeaTorresdey J.L.; Parson J.G.; Gomez E.; Peralta Videa J.; Troiani H.E. and Santiago P. Journal of American Chemical Society. 2002. 2, 397-401.
- Vahabi K.; Mansoori G.A.; Karimi .S, . In science J. 2011.,1,65-79.
- Subramaniam M, Alikunhi NM, Kandaasamy K… Ad SciLett. 2010 3. 428-33. 27.
- Nanda A and Majeed S., Int j pharm pharmsci, 2014., 6,(2), 609-612.

This work is licensed under a Creative Commons Attribution 4.0 International License.









