The Salts of Fatty Acids as Precursors for Preparation of Silver Nanoparticles in Organic Solvents
V. N. Glushko*, Е. E. Anisimova and L. I. Blokhina
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA»), 3, Bogorodskiy val, Moscow, 107076, Russia.
Corresponding Author E-mail: tetrazoli@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/320404
Article Received on :
Article Accepted on :
Article Published : 06 Aug 2016
The silver salts of fatty acids were studied as precursors for the preparation of colloidal dispersions of silver nanoparticles and UHMWPE (ultra-high-molecular-weight polyethylene) composite with silver nanoparticles, as well as the composition, the spectra and SEM (scanning electron microscopy) results.
KEYWORDS:Silver laurate; silver palmitate; ascorbic acid; differential scanning spectroscopy; microscopy; plasmon resonance; ethylene glycol; ethanol; methanol; reduction; polymer matrix
Download this article as:| Copy the following to cite this article: Glushko V. N, Anisimova E. E, Blokhina L. I. The Salts of Fatty Acids as Precursors for Preparation of Silver Nanoparticles in Organic Solvents. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Glushko V. N, Anisimova E. E, Blokhina L. I. The Salts of Fatty Acids as Precursors for Preparation of Silver Nanoparticles in Organic Solvents. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20245 |
Introduction
Interest in the silver nanoparticles and materials with their use is increasing rapidly mainly due to their unusual physical characteristics different from the properties of the corresponding compact materials [1-4].
Colloidal solutions of the silver nanoparticles have an antimicrobial effect with a wide pharmacological spectrum, they can be used for creation of the nanosorbents for producing bactericidal paints and enamels, nanomaterials for creation of antiviral and fungicidal fabric and nonwoven disposable and non-disposable textile materials applied in hospitals, packaging materials that can significantly increase the shelf life of food products [5-6].
Theoretical Analysis
Nowadays, the creation of metal nanoparticles is one of the fastest growing areas of modern nanotechnology, which allows obtaining materials with unique properties.
It is proposed a lot of physical, chemical, biochemical methods for the preparation of colloidal nanosized particles, including cryochemical reduction, vacuum evaporation, and use of pulsed lasers [7].
Metal nanoparticles are lyophobic colloids with low aggregate stability. Their exceptionally high chemical reactivity is based on the evolved interphase, the excess energy of their surface atoms. Calculations have shown that with a decrease in the size of the spherical particles in the three orders of magnitude (from 1 µm to 1 nm), their specific surface area is also increased by three orders of magnitude (from 0.5 to 500 m2/g), and the proportion of surface atoms reaches tens of percent. All this leads to spontaneous processes of nanoparticles aggregation and actualizes the problem of their stabilization [8].
Among the main methods of stabilization are
– functionalization of NP (nanoparticle) surface by groups or protective layers – adsorption of the surfactant with the functional groups of different nature occurs on the NP surface, thiols, carboxylic acids, polyacrylic acid, polydimethylsiloxane, various surfactants, etc. are the most commonly used for these purposes (stabilization in Langmuir-Blodgett films and synthesis of «core-shell» NPs);
-NP localization on the surface of the carriers of different nature – on beads of different nature, with the characteristic size of about hundreds of nanometers.
One of stabilization methods is a matrix isolation, which is used in the form of various types of polymers (organic and inorganic polymers, ceramic – and others) [1, 9, 10].
The literature describes a method for producing monodisperse particles of silver in the thermal decomposition of its carboxylates with a general formula CnH2nO2Ag (n = 6,8,10,12,14,16,18,20,22), which are major components of photo thermally developable materials [11].
The ultimate goal of our research is to study the possibility of admission of silver nanoparticles into the polymer using silver salts of fatty acids as a precursor (silver carboxylates), and therefore, at first we carried out the work to determine the conditions for obtaining the silver nanoparticles by reduction of above mentioned compounds in an organic medium.
Materials and Methods
A series of experiments was carried out in order to investigate the possibility of obtaining colloid solutions of silver nanoparticles by reduction of silver salts of fatty acids, namely silver laurate and silver palmitate, using an organic reducing agent in an organic solvent media in order to determine their properties and stability. These salts are practically insoluble compounds, with the exception of their limited solubility in ethanol and methanol, in which they are dissolved at a concentration of 10-3 – 10-4 M that allows to carry out experiments on their basis in order to obtain the silver nanoparticles.
We obtained silver palmitate and laurate using the general procedure: an aqueous solution of silver nitrate is added to an aqueous acid solution and the precipitate of silver palmitate or laurate salts are filtered off respectively. Preparation of these compounds was confirmed by microanalysis and analysis of the silver content.
Reagents with the following characteristics were used in the experiments
Lauric acid – mp. 44°C; 98.9%
Palmitic acid – mp. 62.9°C; 98.1%
Silver nitrate – 99.9%
Caustic soda – 99.8%
Ethylene glycol – 99.7%
Ethanol – 96.0%
Methanol – 99.9%
Ascorbic acid – 99.9%
Preparation of silver palmitate- C16H31O2Ag and laurate-C12H23O2Ag
0.0375M of NaOH was added to a suspension of 0.02 M of palmitic or lauric acid in 100 cm3 of water at 60°C, heated to 80°C, the resultant solution was cooled to 10°C, filtered off and dried. Yield of sodium salts of carboxylates is 91%.
A solution of 0.02M of sodium salt of palmitic (lauric) acid in 350 cm3 of 7-% ethyl alcohol was heated to 80°C and 0.02M AgNO3 solution in 100 cm3 of water was added. After stirring for 1 hour, the precipitate of silver carboxylate was filtered off.
Identified % Calculated %
Silver laurate
C – 46.93; 46.88 C – 46.92
H – 7.83; 7.87 H – 7.55
Silver palmitate
C – 52.87; 52.46 C – 52.85
H – 8.55; 8.68 H – 8.53
Further studies were carried out to obtain colloidal solutions of silver nanoparticles from such salts.
Method 1
20 cm3 of ethanol was added to 0.0061 g of silver laurate (C = 10-3M) and stirred for 3 hours at 50°C. 10 cm3 of ascorbic acid solution with a concentration of 10-3 M was poured in ethylene glycol. The solution turned yellow. The peak of low intensity with a maximum 415 nm was detected at the plasmon resonance spectrum. Dispersion was stable throughout the day (Fig. 1).
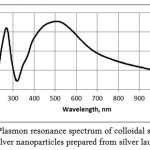 |
Figure 1: Plasmon resonance spectrum of colloidal solution of silver nanoparticles prepared from silver laurate |
Method 2
20 cm3 of methanol was added to 0.0061g of silver laurate (C = 10-3 M) and ascorbic acid solution in ethylene glycol with a concentration of 10-3M. It was stirred for two hours at a temperature of 50°C and 10 cm3 of ascorbic acid solution was poured in ethylene glycol with a concentration of 10-3 M. The solution was pale yellow and turned transparent after 10 minutes, therefore the plasmon resonance spectrum failed to be taken.
Method 3
10 cm3 of ascorbic acid solution in ethanol at a concentration of 10-4 M was poured at room temperature to 10 cm3 of silver laurate solution in ethanol at a concentration of 10-4 M and stirred in magnetic stirrer. Silver aggregated.
Method 4
20cm3 of ascorbic acid in ethylene glycol with a concentration of 10-4 M was poured at room temperature to a solution of silver laurate in 20 cm3 of ethanol with a concentration of 10-4 M and stirred. The reaction mass turned yellow, which indicated the formation of nanoparticles, but after 15 minutes the silver aggregated.
Method 5
10 cm3 of ascorbic acid solution in ethylene glycol (C = 10-3 M) was poured to 10-4 M solution of silver palmitate in 20 cm3 of ethanol while stirring in magnetic stirrer. The solution became yellow. The plasmon resonance spectrum contains an absorption band with a maximum at 420 nm (Fig. 2). The dispersion was stable for one day.
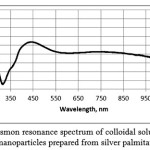 |
Figure 2: Plasmon resonance spectrum of colloidal solution of silver nanoparticles prepared from silver palmitate. |
Method 6
50 cm3 of potassium citrate solution in ethylene glycol with a concentration of 10-3 M was poured to 10 cm3solution of silver palmitate in methanol (C = 10-3 M) while stirring in magnetic stirrer. The mixture was heated for 20 minutes at 65°C. The solution turned yellow and aggregated very quickly.
Results for obtaining dispersions of silver nanoparticles by reduction of fatty acid salts are presented in Table 1.
The method of experiments with silver carboxylates in UHMWPE matrix
Silver laurate 0.0061 g (2·10-4 M) was dissolved in 20 ml of ethanol for 6 hours at 50 °C. The reaction mixture was cooled to room temperature and 0.5 g of UHMWPE was added, then 10 ml of ascorbic acid solution in ethylene glycol with a concentration of 10-3 M was poured while stirring in a magnetic stirrer.
The gray-green reaction mixture was stirred for 4 hours, UHMWPE was filtered and washed with distilled water. The filtrate was colorless, UHMWPE was gray. UHMWPE was dried in an oven at 110°C for an hour, and it turned yellowish.
The filtrate was examined using electron spectroscopy.
Results and Discussions
Thus as a result of studies, it was found that in the preparation of silver nanoparticles from its carboxylates by chemical reduction in non-aqueous media by using ascorbic acid and potassium citrate, in most cases we obtained unstable colloids which aggregated within several minutes. The most stable, at least for one day, were dispersions obtained when we used ascorbic acid solution in ethylene glycol with a concentration of 10-3 M as the reducing agent, and ethanol with a precursor concentration of 10-3 M therein as a solvent for silver carboxylate. In this case, with silver laurate the process runs at a temperature of 50°C and with silver palmitate at room temperature.
Plasmon resonance spectra of the most stable colloidal solutions are presented in Figures 1 and 2.
Table 1 presents the results of research on the preparation of colloid solutions of silver nanoparticles from its carboxylates.
Table 1: Preparation of silver nanoparticles from silver carboxylates
|
Silver salt сoncentra-tion, mol/l |
Solvent |
Reducing agent concentra-tion, mol/l |
Solvent volume, |
λ max, nm |
Stability |
|
Silver laurate 10-3 |
Ethanol 20 ml |
Ascorbic acid 10-3 |
Ethylene glycol 10ml |
505 |
Stable for24 hours |
|
Silver laurate 10-3 |
Methanol 20 ml |
Ascorbic acid 10-3 |
Ethylene glycol 10 |
– |
Aggregates |
|
Silver laurate 10-4 |
Ethanol 10 ml |
Ascorbic acid 10-4 |
Ethanol 10 |
– |
Nanoparticles are not formed |
|
Silver laurate 10-4 |
Ethanol 20 ml |
Ascorbic acid 10-4 |
Ethylene glycol 20 |
410 |
Aggregates in 15 minutes |
|
Silver palmitate 10-4 |
Ethanol 20 ml |
Ascorbic acid 10-3 |
Ethylene glycol 10 |
445 |
Stable for 24 hours |
|
Silver palmitate 10-3 |
Methanol 10 |
Potassium citrate 10-3 |
Ethylene glycol 50 |
– |
Aggregates |
Due to the instability of the dispersions obtained, it was carried out an experiment to obtain silver nanoparticles from silver carboxylates in the polymer matrix by reduction of ascorbic acid using ultrahigh molecular weight polyethylene (UHMWPE) as a polymer matrix at a temperature of 50°C.
Ethylene glycol was usedas a solvent forascorbic acid, and ethanol, methanol and ethylene glycol were used as the solvent for the silver carboxylates. By scanning electron microscopy, it was revealed that when using ethanol as the solvent, spherical shape particles with diameter not exceeding 100 nm (Fig. 3) are evenly distributed on the surface of UHMWPE, when using methanol, the larger particles are evenly distributed in UHMWPE (Fig.4) and a large number of aggregates are obtained in ethylene glycol (Fig. 5).Diffuse reflection spectrum of obtained UHMWPE impregnated with silver nanoparticles is shown in Figure 6.
 |
Figure 3: SEM image of the composite obtained in ethanol Click here to View Figure |
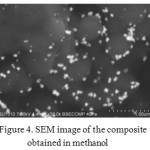 |
Figure 4: SEM image of the composite obtained in methanol Click here to View Figure |
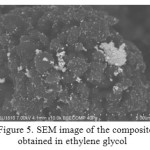 |
Figure 5: SEM image of the composite obtained in ethylene glycol Click here to View Figure |
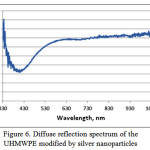 |
Figure 6: Diffuse reflection spectrum of the UHMWPE modified by silver nanoparticles Click here to View Figure |
The silver content in the resulting composites is equal to 0.19 to 0.25 % when using laurate and 0.025 to 0.038 % when using silver palmitate according to standard penetration test on mass spectroscopy.
Conclusion
It is found that, when using chemical reduction of silver carboxylates in non-aqueous media, the dispersion obtained are volatile in organic compounds, aggregating within a short period. Therefore, it can be concluded, that silver nanoparticles from its carboxylates are necessary to obtain by their stabilization in the polymer matrix.
It is preferably to carry out UHMWPE impregnation using silver laurate when dissolving it in ethyl alcohol, as we obtain the composite with a narrow distribution of nanoparticles with size of evenly distributed on the surface of UHMWPE with a high silver content compared to silver palmitate used for this purpose.
Acknowledgments
The applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.576.21.0024 of June 27, 2014. (project № RFMEFI57614X0024).
References
- Pomogailo, A.D.; Uflyand, I.E. Metal nanoparticles in polymers.M.: Chemistry.2000, 672
- Bespalov, A.V.; Buiklisky V.D. Chemistry and Chemical Engineering.2012,55(3), 59-61
- Panacek,А.; Kvitek, L.; Prucek, R.; Kolar, M. J. Am. Chem. Soc. 2006,26(16), 37-43
- Kosobudsky, I.D.; Kulbatsky D.M.; Muzalev, P.A.Chemistry and Chemical Engineering.2011,54(4), 97-100
- Taylor, M. R.Washington: Woodrow Wilson International Center for Scholars, 2008, 100
- Krutyakov, Yu. A.; Kudrinskiy, A. A.; Olenin A. Yu.Russian Chemical Reviews. 2003, 77(3), 242-269
- Peng, K; Qian, K; Chen, W.Mater.Sci. Eng., A, 2004, 379, 378-383
CrossRef - Litmanovich, O.E. High molecular compounds.Series C.2008,50(7), 1370
- Xu, J.; Han, X.; Liu, H.; Hu, Y. Colloids Surf.A,2006,273, 179.
CrossRef - Zana, R. Adv.Colloid Interface Sci.2002,97, 205.
CrossRef - Sharafutdinov, M.R. Preparation and research using in situ methodfor synchrotron radiation diffraction of order structures of silver nanoparticles by thermal decomposition of its carboxylate, diss.k.h.n., Novosibirsk, 2009, 105.

This work is licensed under a Creative Commons Attribution 4.0 International License.









