The Effect of Calcination Temperature on Synthesis of B4C-Nano Tib2 Composite by Co-Precipitation Method
Saeid Abedini Khorrami1, Hamid Reza Baharvandi2 and Roshanak Lotfi1
1Department of Chemistry, North Tehran Branch, Islamic Azad University, Tehran, Iran.
2Department of Material, Malek Ashtar University of Technology, Tehran, Iran.
*Corresponding Author E-mail: s_akhorrami@iau-tnb.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/320457
Titanium diboride is one of the candidate materials for high temperature applications and also for control rod elements in high temperature reactors. This paper presents the experimental data on the composites of B4C-nano TiB2 that were synthesized successfully by co-precipitation method at temperatures between 973 and 1523K. Titanium tetraisopropoxide, boron carbide and isopropanol were used as the precursor materials. The phase constitution and microstructure of B4C-nano TiB2 during synthesis were investigated. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to determine phase and microstructure of TiB2-B4C composites. The DTA/DDTA and TG/DTG results improve that the first exothermic reaction is TiO2 phase and second exothermic reaction takes place at 1523K which is TiB2 phase
KEYWORDS:B4C-nano TiB2; DTA/DDTA; TG/DTG; XRD
Download this article as:| Copy the following to cite this article: Khorrami S. A, Baharvandi H. R, Lotfi R. The Effect of Calcination Temperature on Synthesis of B4C-Nano Tib2 Composite by Co-Precipitation Method. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Khorrami S. A, Baharvandi H. R, Lotfi R. The Effect of Calcination Temperature on Synthesis of B4C-Nano Tib2 Composite by Co-Precipitation Method. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20779 |
Introduction
As one of the hardest materials known, boron carbide ranks third behind diamond and cubic boron nitride. Boron carbide (B4C) ceramics possesses excellent mechanical and physical properties of good impact and wears resistance, hardness and high melting, excellent resistance to chemical materials as well as high capability for neutron absorption [1-6]. Moreover the low density of B4C and its high Young’s modulus recommend this material for the construction of light-weight armor such as in the helicopter and similar aero-application [7] However, the widespread application of B4C ceramics has been restricted because of the poor sinterability due to a low self-diffusion coefficient and the low fracture toughness (< 2.2 MPa m1/2) [8, 9].
Titanium diboride with high melting point (3498 K), high hardness (25 GPa), low density (4.5 g cm-3), high electrical conductivity (22× 106 Ω cm), good thermal conductivity (96 W/mK) and considerable chemical stability is one of the candidate materials for high temperature structural and wear application [10, 11]. Since both B4C and TiB2 have high hardness and high melting points as well as chemical stability at elevated temperature, the B4C-TiB2 composites were expected to be used for advanced structural materials.
TiB2 can be prepared by carbothermic reduction of mixed oxides of titanium and boron, reduction of mixed oxides by metals like aluminum, magnesium and silicon, reduction of titanium oxide by boron carbide and carbon or synthesized from the elements by heating, mechanical alloying or self-propagating high temperature synthesis [12-14], pulsed electric current sintering [15], mechanochemical synthesis [16], dc magnetron sputtering [17] and milling assisted sol-gel [18-21].
Being intrinsically brittle, B4C often requires some additive to improve its mechanically properties and sintering behavior. Numerous researchers have shown that the addition of TiB2 to B4C can decrease the porosity level and improve the fracture toughness as well as flexural strength [22]. Recently, some researchers of B4C based-composites such as B4C/CrB2, B4C/Al, B4C/TiC and B4C/ZrB2 have been carried out [23]. It has been considered that the additions of secondary phases to B4C matrix can improve its mechanical properties [24].
In the present study, B4C-nano TiB2 composites with 10 ww% TiB2 were obtained by in situ synthesis from boron carbide, titanium tetraisopropoxide and isopropanol. The effect of calcination temperature on the size, morphology and phase were discussed. Titanium diboride was synthesized by co-precipitation method. Also, thermogravimetric study on the formation of TiB2 by boron carbide reduction of TiO2 was carried out in our laboratory.
Experimental
Boron carbide (95%, B4C, Merck), titanium tetraisopropoxide (97%, TTIP, Alfa Aesar), isopropanol (99.6%, Merck) were used to synthesis. All materials were used without further purification, but were dried in an oven at 423K to remove the moisture content before use. Boron carbide contains 5.0 ww% phenolic resin which used as carbon source. Deionized water was used for all experiments. The precursor powders were obtained by using co-precipitation method. TiB2 was prepared by the reaction given below:
2TiO2 + B4C+ 3C → 2TiB2 + 4CO (1)
Titanium tetraisopropoxide (1.15 mol) and boron carbide (18.1 mol) were dissolved in isopropanol (solution A). Also, boron carbide was dissolved in deionized water and isopropanol (solution B). Solution B was gradually added to solution A. The prepared mixture was stirred and heated at 298K for 4h. In this work, B4C-nano TiB2 composites are contained 10 ww% TiB2.
The X-ray diffraction pattern (XRD, X’Pert MPD, Philips, Holand) and scanning electron microscopy (SEM, XL-30, Philips, Holand) of initial B4C is shown in Figures 1 and 2, respectively. The crystalline phase during the reaction was investigated by an X-ray diffractometer using Cu-Kα radiation (40 kV, 40 mA). The mixture of TTIP, B4C and isopropanol was placed at 423-433K.
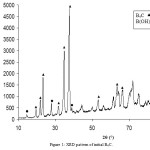 |
Figure 1: XRD pattern of initial B4C. |
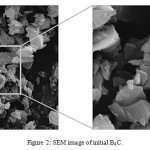 |
Figure 2: SEM image of initial B4C. |
Then, the powder were milled and after that, the XRD pattern and SEM were used to determined of particles size and to study of morphology that are illustrated in Figures 3 and 4, respectively. The prepared powder were placed to determine calcination temperature at 973, 1273 and 1523K for 60 minutes with 150 sccm (denotes cubic centimeter per minute at STP) argon flow.
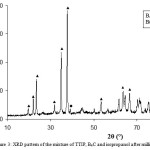 |
Figure 3: XRD pattern of the mixture of TTIP, B4C and isopropanol after milling at about 430K.
|
 |
Figure 4: SEM image of the mixture of TTIP, B4C and isopropanol after milling at about 430K. |
Figure 5 presents the DTA/DDTA and TG/DTG plots of the formation of titanium diboride.
 |
Figure 5: DTA/DDTA and TG/DTG curves of the formation of titanium diboride for a temperature up to 1700K.
|
The composition and nanostructure of the B4C-TiB2 composites in 1273 and 1523K were shown by SEM in Figure 6.
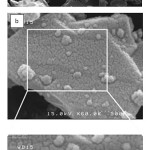 |
Figure 6: SEM image of the B4C-nano TiB2/TiO2 composite at: (a) 1273 and (b) 1523K. Click here to View table |
Also, XRD patterns of B4C-TiB2 composites at 937, 1273 and 1523K for 60 minutes with 10 ww% of TiB2 are shown in Figure 7 that is used to determined for composites phases.
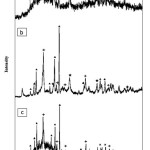 |
Figure 7: Diffractographe showing the comparative effects associated at different temperature of introducing the mixture: (a) 973,(b) 1273 and (c) 1523K. |
Results and discussion
The prepared powder for formation of B4C-nanoTiB2 composites with 10 ww% TiB2 are placed to determine calcination temperature at 973, 1273 and 1523K with argon flow. The DTA/DDTA and TG/DTG plots in Figure 5 show that the first exothermic reaction is associated with many weight loss leading to dehydration and evaporation of water from Ti(OH)4 phase and the formation of TiO2 phase by a decrease in mass of about 13.2 wt% that is given by below reaction:
Ti(OH)4 → TiO2 + 2H2O (2)
second exothermic reaction takes place at 1523K which also in this reaction is associated with weight loss about 4.39 wt% that leading to the formation of TiB2 nanopowder from TiO2 particles that is given by
TiO2 + 0.5 B4C + 1.5 C → TiB2 + 2CO (3)
Figure 6(a) shows the SEM image of B4C-nano TiO2/TiB2 composites at 1273K. Particles size at 1273K is between 10-70 nm. FE-SEM image indicate the determination of nanoparticle sizes and morphology of TiB2 on B4C at 1523K are shown in Figure 6(b). In the SEM of samples, the distribution of the B4C and nano TiB2 was not uniform. The dark elongated grains are B4C, whereas the bright irregular or equiaxed grains are TiB2. TiB2 nanoparticles size at 1523K is between 10-40 nm. Figure 7(a) shows the XRD pattern of composite at 937K. In this calcination temperature exist B4C and TiO2 phases that is meaning this temperature is not enough to convert TiO2 to TiB2. The XRD pattern of composite at 1273K shows that three phases of B4C, TiO2 and TiB2 exist but calcinations temperature should be increased. Figure 7(c) confirms that only TiB2 and B4C phases exist at 1523K that is enough to convert TiO2 to TiB2. Pure B4C-nano TiB2 phases were detected with no peaks of unreacted TiO2 and Ti(OH)4, thus indicating the full conversion of reagents into products was achieved according to reaction (3). Therefore, results indicate that synthesis of B4C-nano TiB2 from calcinations process of mixed powder is achieved at temperature higher than 1523 K. The XRD patterns of the fractured and the ground surfaces of the composites at 973, 1273 and 1523K are compared in Figure 7. The JCPDS card matching the spectra is 86-1129 for rhombohedral B4C, 84-1286 for anataze TiO2 and 85-2083 for hexagonal TiB2. Since the C source is amorphous carbon black only TiO2 and B4C phases were found in the starting powder. The XRD pattern reveals full conversion of TiO2 to hexagonal TiB2 after 60 minutes at 1523K. Calcinations process at lower temperature than 1523K contain B4C, TiO2 and TiB2 powders. Decreasing of particles size take place because of suitable selection of optimum calcinations and time for growth of TiB2 nano particles.
Conclusions
Titanium diboride was synthesized successfully by co-precipitation method using TTIP, B4C and isopropanol. In this study, nano TiB2 particles in three steps produce on microstructure surfaces B4C at three calcination temperature. In the first reaction, TTIP converted to Ti(OH)4. Then, TiO2 formed from Ti(OH)4 at 553K. Finally, TiO2 was transferred TiB2. The fracture surfaces of the powder were observed by FESEM. The micrographs, reported in Figure 6 confirmed the complete densification of the nanostructured powders. The XRD and SEM results are proved which two phases of B4C and nano TiB2 with 10 ww% at 1523K. The nanoparticle sizes of the synthesized nano TiB2 on surface of B4C microstructures were found between 10-40 nm.
References
- Chen, M. W.; Mccauley, J. W.; Lasalvia, J. C. J. American Ceram. Soci.2005, 88, 1935-1942.
CrossRef - Roy, T. K.; Subramanian, C.; Suri, A. K. Ceram. Int.2006, 32, 227-233.
CrossRef - Rutkowski, P. Compos. Theory and Prac. 2013, 13, 33-39.
- Hayun, S.; Rittel, D.; Frage N. Mater. Sci. Engin. A2008, 487, 405-409.
CrossRef - Fanchini, G.; Gupta, V.; Mann, A. B. J. American Ceram. Soci.2008, 91, 2666-2669.
CrossRef - Srivatsan, T. S.; Guruprasad, G.; Black, D.; Radhakrishnan, R.; Sudarshan, T. S. Powder Technol,2005, 159, 161-167.
CrossRef - Deng, J.; Sun, J. Ceram. Int.2009, 35, 771-778.
- Adrian, G.; Yehoshua, Y.; Ayala, G. J. Europ. Ceram. Soci.2007,27,695-700.
CrossRef - Gopal Krishna, U. B.; Sreenivas Rao, K. V.; Vasudev, A. B.Inter. J. Metallur. & Mater. Sci. and Engin. 2013, 3, 41-48.
- Pezzotta, M.; Zhang, Z. L.; Jensen, M.; Grande, T.; Einarsrud, M. A. Computational Mater. Sci. 2008, 43, 440–449.
CrossRef - Licai, F.; Jun, Y.;Qinling, B.;Weimin, L.Nanoscale Res. Lett.2009, 4, 11–16.
CrossRef - Tang, W.;Zheng, Z.; Wu, Y.; Wang, J.; Lu, J.;Liu, J. Transactions of Nonferrous Met. Soci. of China. 2006,16,613-617.
CrossRef - Deorsoia, F. A.; Atias Adrian, I. C.; Ortigoza Villalba, G. A.; Debenedetti, B. Mater Research Bull. 2011, 46, 995-999.
CrossRef - Aminikia, B. Powder Technol.2012, 232, 78-86.
CrossRef - Huang, S. G.; Vanmeensel, K.; Vander Biest, O.; Vleugeis, J. J. Europ. Ceram. Soci.2011, 31, 637-644.
CrossRef - Aviles, M. A.; Cordoba, J. M.; Sayagues, M. J.; Gotor, F. J. Ceram. Int.2011, 37, 1895-1904.
CrossRef - Sanchez, C. M. T.; Rebollo Plata, B.; Maiada Costa, M. E.; Freire, F. L. Surf. and Coat. Technol. 2011, 205, 3698-3702.
CrossRef - Rabiezadeh, A.; Ataie, A.; Hadian, A. M. Int. J. Refractory Metal. and Hard Mater.2012, 33, 58-64.
CrossRef - Nemeryuk, A. M.; Lylina, M. M. Orient J. Chem.2015, 31, 2415-2420.
CrossRef - Glushko, V. N.; Sadovskaya, N. Y.; Usova, O. A.; Blokhina, L. I.; Kozhukhov, V. I. Orient J. Chem.2015, 31, 2515-2520.
CrossRef - Nemeryuk, A. M.; Lylina, M. M. Orient J. Chem. 2015, 31, 2481-2486.
CrossRef - Lin, Q.; Dong, S.; He, P.; Zhou, H.; Hu, J. J. Inorganic Mater. 2015, 30, 667-672.
CrossRef - Hongkang, W.; Yujun, Z.; Xiangyu, D. J. Ceram. Process Resea. 2011, 12, 599-601.
- Du, B.; Paital, S. R.; Dahotre, N. B. Optics & Laser Technol.2013, 45, 647-563.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









