Physicochemical Evidence for the Adsorption of Extract of Jacaranda Mimosifolia on Steel Against Corrosion in Acid Medium
Kavitha Rose1,2, Monikandon Sukumaran3, Kesavan Devarayan1,4,*, Gopiraman Maya krishnan5 and Sankar Arumugam2,*
1Department of Chemistry, Dhirajlal Gandhi College of Technology, Salem, India.
2Department of Chemistry, Kandasamy Kandar’s College, Salem 638182, India.
3Department of Basic Engineering, College of Fisheries Engineering, Tamil Nadu Fisheries University, Nagapattinam 611 001, Tamil Nadu, India.
4Department of Basic Sciences, College of Fisheries Engineering, Tamil Nadu Fisheries University, Nagapattinam 611 001, Tamil Nadu, India.
5Department of Applied Bioscience, College of Life and Environment Science, Konkuk University, Seoul, South Korea.
*Corresponding Author E-mail: dev.kesavan@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320444
For the first time, the homogeneous adsorption of ethanolic extract of Jacaranda mimosifolia green inhibitor is evaluated for inhibition of steel corrosion in acid environment. The inhibitive properties are evaluated by means of weight loss measurements, polarization studies, and electrochemical impedance spectroscopy. A maximum of 96% of inhibition efficiency is achieved by using 500 ppm of the green inhibitor. The mechanism and nature of adsorption of inhibitor on the surface of steel are evidenced by Langmuir adsorption isotherm and energy dispersive X-ray spectral elemental mapping.
KEYWORDS:Adsorption; Elemental Mapping; Evidence; Jacaranda mimosifolia; Green Inhibitor; Steel
Download this article as:| Copy the following to cite this article: Rose K, Sukumaran M, Devarayan K, krishnan G. M, Arumugam S. Physicochemical Evidence for the Adsorption of Extract of Jacaranda Mimosifolia on Steel Against Corrosion in Acid Medium. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Rose K, Sukumaran M, Devarayan K, krishnan G. M, Arumugam S. Physicochemical Evidence for the Adsorption of Extract of Jacaranda Mimosifolia on Steel Against Corrosion in Acid Medium. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20968 |
Introduction
Steel corrosion in acidic solutions has become an intensive academic research topic since it can greatly influence the economy of any country. It has been reported by several researchers that steel corrosion can be controlled by the use of suitable inhibitors. Inorganic inhibitors such as phosphates, chromates, and arsenates were found to be effective against the corrosion of steel in acidic medium. However, the major disadvantage of using these inorganic inhibitors is their toxicity and non-ecofriendliness. Organic inhibitors are one of the alternatives to inorganic inhibitors. It is well known that the first step of inhibitive action of an inhibitor is adsorption of the additive on to the metal surface. For this purpose, inhibitors with polar functions with heteroatoms such as nitrogen, oxygen, and/or sulphur preferable1.
In recent years, inhibitors of plant origin have exhibited excellent inhibition properties against the steel corrosion1,2. Inhibitors from natural resources such as plants are interesting since they are abundant, economical, and environmentally friendly. In order to explore the efficient organic inhibitors, the authors have studied several synthetic as well as plant-based green inhibitors against the corrosion of steels in different media3-6. In an effort to contribute to the current interest on development of the ecofriendly inhibitors, in this study, the authors examined the ethanolic leaf extract of Jacaranda mimosifolia (JM) against the corrosion of steel in 1.0 M HCl. JM (Sakaranda in Tamil) belongs to Bignoniaceae family and an habitat of tropical areas such as India, South America, Brazil, and South Africa. Different parts of the JM were evaluated for applications such as trace metal and dye adsorption from contaminated water7-9. In view of abundance, environmental friendliness, and economy, in the present study, the ethanolic extract of JM is evaluated as an active steel corrosion inhibitor in an acid medium. The inhibition efficiency of JM extract is studied by means of gravimetric weight loss and electrochemical studies. The energy dispersive X-ray spectroscopic measurement also used to observe the surface of the inhibitor adsorbed-steel.
Experimental
Preparation of Inhibitor
The green inhibitor was prepared by a procedure similar to our previous reports8,9. In brief, the shade-dried leaves of JM (1 kg) were powdered finely and soaked in 95% ethanol for 3 days. Then the solvent was removed under vacuo to obtain oily precipitate (26 g).
Preparation of Test Solutions
1.0 M HCl solutions were prepared using concentrated HCl (Merck, AR) using double distilled water. The concentration range of the JM extract was 100-500 ppm. The acid solution without inhibitor solution is taken as blank solution. The acid solutions were prepared freshly just before each experiment.
Weight Loss Studies
The chemical composition of the steel specimens used in this study was C, 4.93%; Mn, 1.09%; Si, 1.78%, and the remainder being Fe. At first, the specimens were abraded using emery sheets of different grades and then washed in distilled water and degreased in acetone followed by drying at room temperature. The weight of the specimen was noted before (W0) and after (W) blank experiment using 1.0 M HCl and the experiments were performed in triplicates. Using the following equations 1-3, the inhibition efficiency (IE%), surface coverage (q), and corrosion rates (CR) were calculated.

Where, CR – corrosion rate (mm/y), W0 – weight difference (g) before and after weight loss measurements; A – exposed area of the specimen, t – immersion time (h), and D – density of the specimen (g/cm3). The weight loss values were used to plot adsorption isotherms such as Langmuir adsorption isotherm and to calculate the thermochemical parameters.
Electrochemical Studies
Electrochemical experiments were carried out in a conventional three electrode cell assembly with steel having 1 x 1 cm2 area as a working electrode, platinum as a counter electrode, and a saturated calomel electrode as reference. The thermodynamic Tafel polarization and electrochemical impedance spectroscopic (EIS) measurements were carried out using CH6043b electrochemical analyzer. The EIS and Tafel polarization measurements were performed similar to our previous studies8,9.
Surface Analysis and Elemental Mapping
The surface morphology and energy dispersive X-ray spectral analysis (EDS) of specimens after treatment in blank solution and with inhibitor were observed under a JSM-5900 scanning electron microscopy coupled with EDS.
Results and Discussion
Weight Loss Studies
The weight loss data shown in Fig.1 and listed in Table 1. The JM extract concentration was varied from 100-500 ppm. At the highest concentration of 500 ppm, the inhibitor has a highest inhibition efficiency of 92.1%. This is attributed to the surface coverage of steel by the inhibitor molecules present in the JM extract. It is interesting to note that no significant inhibition was observed above 500 ppm of the inhibitor. This may be attributed to the saturation of the surface coverage due to the presence of large quantities of inhibitor molecules that could not influence the adsorption process.
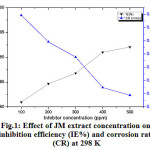 |
Figure 1: Effect of JM extract concentration on inhibition efficiency (IE%) and corrosion rate (CR) at 298 K |
Table 1: Corrosion parameters for inhibition of steel corrosion in 1.0 M HCl by JM extract at 298 K
| C (ppm) | Surface Coverage |
IE% | CR (mm/y) | Kads (kJ/g) | DGads (kJ/g) |
| 0 | – | – | 2.718 | – | – |
| 100 | 0.810 | 80.952 | 0.547 | 42.5 | -19.4 |
| 200 | 0.847 | 84.656 | 0.446 | 27.6 | -18.3 |
| 300 | 0.868 | 86.772 | 0.388 | 21.9 | -17.7 |
| 400 | 0.910 | 91.005 | 0.273 | 25.3 | -18.1 |
| 500 | 0.921 | 92.063 | 0.244 | 23.2 | -17.9 |
Adsorption Isotherm
Generally, the inhibition of corrosion using inhibitor molecules proceeds via either physical adsorption or chemical adsorption or both. It is imperative to understand the nature of the adsorption behavior of the inhibitor molecules. In this view, the data from weight loss measurements were evaluated to fit for different isotherms such as Langmuir, Freundlich, and Frumkin adsorption isotherms. Linear relationship was observed for the data only in the case of Langmuir adsorption isotherm (Fig.2). The Langmuir adsorption isotherm can be understood from the relationship between surface coverage and the inhibitor concentration as shown in the following equation (4).

Where, Kads is the equilibrium constant of adsorption process.
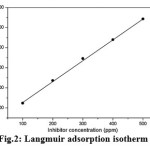 |
Figure 2: Langmuir adsorption isotherm
|
The correlation co-efficient (R2) for the Langmuir isotherm was 0.9995. The result indicated that the adsorption mechanism of the corrosion inhibition process in the present study obeys Langmuir adsorption isotherm. In other words, corrosion of inhibition of steel by JM extract predominantly proceeds via physical adsorption. The higher inhibition efficiency of the JM extract is attributed to the molecules present in the extract. Generally, plant extracts contain alkaloids and flavonoids1,7 which possess aromatic rings and highly electronegative atoms such as oxygen, sulphur, and/or nitrogen in the chemical structure.
Further the data obtained from weight loss studies were subjected to equations (4) and (5) to calculated the equilibrium constant (Kads) and the free energy of adsorption (∆Gads) (Table 1).
∆Gads=-RT ln (55.5Kads) (5)
where R is the gas constant, T is the temperature, and the value of 55.5 is the concentration of water in the inhibitor solution. The values of ∆Gads for all the concentration of the inhibitor was found to between -17.7 and -19.1 kJ/g. Generally, if the ∆Gads is lesser than -20.0 kJ/g then the inhibition is ascertained to physical adsorption process. If the ∆Gads is greater than -40 kJ/g then the inhibition is due to the chemical adsorption of the inhibitor molecules on the surface of the steel. In the present study, the ∆Gads values are lesser than -20.0 kJ/g which clearly indicates that the inhibition mechanism predominantly involved in physical adsorption11-13.
Electrochemical Studies
The Tafel polarization and the EIS studies were performed both in presence and absence of different concentrated solutions of JM inhibitor in 1 M HCl at 298 K. The steady-state was achieved within 2 h. Depressed-EIS semi circles were observed for both experiments with and without 500 ppm of inhibitor (Fig.3a). The charge-transfer resistance values for blank and blank + 500 ppm inhibitor were 11.1 and 211 ohm/cm2, respectively (Table 2). The diameter of the EIS semi-circle for blank + 500 ppm inhibitor increases due to the surface coverage of steel by inhibitor molecules which prevents the attack of the HCl and thus decreases the conductivity. In addition, the values of double layer capacitance (Cdl) were also calculated using the following equation (5).

It is interesting to note that the Cdl values of blank solution (267.4 µF cm-2) are higher than the solution with 500 ppm of inhibitor (7.3 µF cm-2). This phenomenon can be explained by the adsorption of the inhibitor molecules on the steel surface. The adsorption of inhibitor molecules decreases the dielectric constant of steel by covering the surface which obviously led to the decrease in the double layer thickness14.
Table 2: EIS parameters of steel specimen in presence and absence of JM inhibitor in 1.0 M HCl at 298 K
| Inhibitor | Concentration (ppm) | Rct | fmax | Cdl | Ɵ | IE% |
| (Ω cm2) | (µF cm-2) | |||||
| Blank | 0 | 11.1 | 5.38 | 267.4 | – | – |
| JM | 500 | 211 | 103 | 7.3 | 0.948 | 94.8 |
The Tafel polarization results are presented in Fig. 3b and Table 3. The polarization curves indicated that the corrosion current values obtained for JM inhibitor were lower than the blank solution. Further, the Ecorr value between the blank and the JM inhibitor was more than ±85 mV. This is ascertained to the behavior of the inhitor to suppress predominantly anodic reaction15,16.
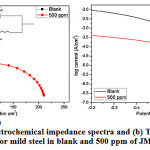 |
Figure 3: (a) Electrochemical impedance spectra and (b) Tafel polarization curve for mild steel in blank and 500 ppm of JM inhibitor
|
Table 3: Tafel polarization parameters of steel specimen in presence and absence of JM inhibitor in 1.0 M HCl at 298 K
| Inhibitor | Concentration (ppm) | bc (mV/decade) | ba (mV/decade) | –Ecorr | Icorr | Ɵ | IE% |
| (V vs SCE) | (µA/cm2) | ||||||
| Blank | – | 5.687 | 4.292 | 0.562 | 673.3 | – | – |
| Blank+JM | 500 | 5.427 | 4.639 | 0.674 | 27.9 | 0.959 | 95.9 |
Surface Analysis
The microscopic images and ED spectra of the abraded steel specimen, steel treated in blank solution as well as blank solution with 500 ppm of inhibitor are shown in Fig. 4. The elemental composition of these samples obtained from ED spectra are given in Table 4. Fig.4a exhibits a steel surface with scratches caused by manual polishing. Whereas, the acid-treated steel (Figure 4c) exhibits high corrosion along with the corrosion products. The corrosion products are evidenced by ED spectra shown in Figure 4d. Meanwhile the steel treated in acid+inhibitor solution exhibited mostly smooth surface (Fig. 4e). The corresponding ED spectrum is revealed the presence of elements such as O and N which is evident for the deposition of inhibitor molecules on the surface of the steel.
Table 4: Elemental composition of steel specimens
| Sample |
% Composition |
||||||
| Fe | O | C | Mn | Si | Cl | N | |
| Fresh steel specimen | 92.20 | 0 | 4.93 | 1.08 | 1.79 | 0 | 0 |
| Blank | 67.11 | 23.29 | 4.38 | 0.77 | 1.29 | 3.16 | 0 |
| 1.0 M HCl + JM extract | 41.29 | 14.88 | 24.92 | 0.44 | 0.31 | 0.67 | 17.49 |
 |
Figure 4: SEM and ED spectrum of (a, b) raw steel specimen, (c, d) steel treated in 1.0 M HCl, and (e, f) steel treated in inhibitor solution
|
In order to further understand and visualize the adsorption behavior of the inhibitor, the surface of the steel treated in acid + inhibitor is subjected to X-ray elemental mapping (Fig. 5). The results showed that the elements O and N are homogeneously distributed on the surface of the mild steel. This is a direct physicochemical evidence for the adsorption of the JM green inhibitor on the steel surface.
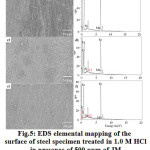 |
Figure 5: EDS elemental mapping of the surface of steel specimen treated in 1.0 M HCl in presence of 500 ppm of JM inhibitor solution
|
Conclusion
For the first time the homogeneous adsorption of the ethanolic extract of Jacaranda Mimosifolia green inhibitor is evaluated and visualized. The efficiency of the inhibitor is evaluated by different methods including gravimetric weight loss and electrochemical studies. The thermodynamic parameters and the Langmuir adsorption isotherm indicated that the inhibitor acts via physical adsorption mechanism. Further, energy dispersive X-ray spectral mapping indicated that the inhibitor molecules are homogeneously distributed on the surface of the steel.
Acknowledgement
Kesavan Devarayan and Monikandon Sukumaran thank College of Fisheries Engineering, Tamil Nadu Fisheries University for motivation towards research. Kavitha Rose thanks the management of Dhirajlal Gandhi College of Technology for their encouragement for research. Sankar A and Kavitha R as well thank the management of Kandasamy Kandar’s College for their support for research.
References
- Raja, P.B.; Mathur, G.S. Mater. Lett. 2008, 62, 113-116
CrossRef - Kesavan, D.: Gopiraman, M.; Sulochana, N. Chem. Sci. Rev. Lett. 2012, 1, 1-8
- Kesavan, D.; Muthu Tamizh, M.; Gopiraman, M.; Sulochana, N.; Karvembu, R. J. Surfact. Deterg. 2012, 15, 567–576
CrossRef - Kesavan, D.; Muthu Tamizh, M.; Sulochana, N.; Karvembu, R. J. Surfact. Deterg. 2012, 15, 751–756
CrossRef - Rajeswari, V.; Kesavan, D.; Gopiraman, M.; Viswanathamurthi, P. J. Surfact. Deterg. 2013, 16, 571-580
CrossRef - Kesavan, D.; Parameswari, K.; Lavanya, M.; Beatrice, V.; Ayyannan, G.; Sulochana, N. Chem. Sci. Rev. Lett. 2014, 2, 415-422
- Trevino-Cordero, H.; Juarez-Aguilar, L.G.; Mendoza-Castillo, D.I.; Hernandez-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Moran, M.A. Ind. Crops Prod. 2013, 42, 315-323
CrossRef - Kavitha, R.; Byoung-Suhk, K.; Kalyanaraman, R.; Sankar, A.; Kesavan, D. J. Mol. Liq. 2016, 214, 111-116
CrossRef - Kavitha, R.; Kesavan, D.; Sankar, A. Chem. Sci. Rev. Lett. 2014, 3, 200-209
- ASTM-G31-72, (2004) Standard practice for laboratory immersion corrosion testing of metals. Annual book of ASTM Standards, Philadelphia
- Gopiraman, M.; Selvakumaran, N.; Kesavan, D.; Karvembu, R. Prog. Org. Coat. 2012, 111, 104-111
CrossRef - Tang, L.; Li, X.; Mu, G.; Li, L.; Liu. G. Appl. Surf. Sci. 2006, 252, 6394-6401
CrossRef - Ali, S.A.; Al-Muallem, H.A.; Saeed, M.T.; Rahman, S.U. Corros. Sci. 2008, 50, 664-675.
CrossRef - Quartarone, G.; Bonaldo, L.; Tortato, C. Appl. Surf. Sci. 2006, 252, 8251-8257.
CrossRef - Hong, J.H.; Lee, S.H.; Kim, J.G.; Yoon, J.B. Corros. Sci. 2012, 54, 174–182.
CrossRef - Riggs, O.L. Corrosion Inhibitors, 2nd ed., C. Nathan, Houston, TX, (1973) 43.

This work is licensed under a Creative Commons Attribution 4.0 International License.









