Novel and Improved Method for the Synthesis of 2-Mercaptobenzimidazole Derivatives
A. El Kihel1*, H. Ait Sir1, S. Jebbari1, M. Ahbala1, S. Guesmi2 and P. Bauchat3
1Laboratoire de chimie bioorganique, Faculté des Sciences, BP20, 24000, El Jadida, Maroc.
2Laboratoire de Chimie de Coordination et d’Analytique, Faculté des Sciences, BP20, 24000, El Jadida, Maroc.
3Université de Rennes1, Sciences Chimiques de Rennes U.M.R.6226, ICMV, Campus de Beaulieu, Avenue Général Leclerc, 35042, Rennes, France.
Corresponding Author E-mail: abdellatifelkihel@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320405
Article Received on :
Article Accepted on :
Article Published : 06 Aug 2016
2-mercaptobenzimidazole derivatives were synthesized by reaction of o-phenylenediamines with N-aminorhodanine. This reaction represent a new synthesis of 2-mercaptobenzazole. The structure of the obtained products was established by spectroscopic data.
KEYWORDS:2-mercaptobenzimidazole; o-phenylenediamines; N-aminorhodanine
Download this article as:| Copy the following to cite this article: Kihel A. E, Ait Sir H, Jebbari S, Ahbala M, Guesmi S, Bauchat P. Novel and Improved Method for the Synthesis of 2-Mercaptobenzimidazole Derivatives. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Kihel A. E, Ait Sir H, Jebbari S, Ahbala M, Guesmi S, Bauchat P. Novel and Improved Method for the Synthesis of 2-Mercaptobenzimidazole Derivatives. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20229 |
Introduction
Thiols are very useful building blocks for the synthesis of various organosulfur compounds: they have several applications in organic synthesis, in bioorganic, medicinal and heterocyclic chemistry [1]. Also, in addition to that, thiols can act as safety-catch linker in peptide chemistry [2]. Morever, thiols have been employed as sulfur-based ligands in transition metal complexes[3, 4]. In this respect, a number of synthetic methods for the preparation of thiol derivatives by the reaction of o-phenylendiamine with carbon disulphide [5, 9], thiourea [10, 11], O-isopropylxanthic acid potassium salt [12], 5-phenyl-1,3,4-oxadiazole-2(3H)-thione derivatives and N-phenylisothiocyanate [13], thiocyanic acid ammoniac salt [14]. In continuation of our research concerning benzimidazoles[15, 19]. The condensation of o-phenylenediamine with N-aminorhodanine was carried out.
Experimental Section
General Procedure
O-phenylenediamines (0.065 mol) was heated with N-aminorhodanine (0.065 mol) in xylem (50 ml) for 5 hours. The obtained residue was filtered and was crystallized from aqueous alcohol (charcoal). The obtained solid was recrystallized in ethanol.
2-mercaptobenzimidazole 3a
Yield = 87 %; mp>250°C.1HNMR (DMSO-d6): 7.10 (m, 2Har); 7.27 (m, 2Har); 12.42 (s,
NH).13CNMR (DMSO-d6): 119.43 (CH); 126.36 (CH); 138.82 (C); 167.12 (C=S). HRMS, m/z: 150(M), calcd for C7H6N2S: 150.02517, found: 150.0251.
2-mercapto-5-nitrobenzimidazole 3b
Yield = 81 %; mp>250°C.1HNMR (DMSO-d6): 7.12 (d, JAB= 8.3Hz, 1Har); 7.29 (q, JBX=2.1Hz, JAB= 8.3Hz, 1Har); 7.46 (d, JBX= 2.1Hz, 1Har); 12.03 (s, NH).13CNMR (DMSO-d6):108.24 (CH); 113.46 (CH); 125.70 (CH); 137.45 (C); 141.94 (C); 148.01 (C); 167.54 (C=S).HRMS, m/z: 195(M), calcd for C7H5N3O2S: 195.01025, found: 195.0102.
2-mercapto-5-methylbenzimidazole 3c
Yield = 83 %; mp>250°C. 1HNMR (DMSO-d6): 2.21(s, CH3); 6.43 (d, JAB= 8.3Hz, 1Har); 6.83 (q, JBX=1.7Hz, JAB= 8.3Hz, 1Har); 7.05 (d, JBX= 1.7Hz, 1Har); 12.03 (s, NH). 13CNMR (DMSO-d6): 21.31 (CH3); 109.50 (CH); 109.99 (CH); 123.49 (CH); 130.69 (C); 131.92 (C); 132.95 (C); 168.12 (C=S). HRMS, m/z: 164(M), calcd for C9H8N2S: 164.04082, found: 164.0408.
5-chloro-2-mercaptobenzimidazole 3d
Yield = 79 %; mp>250°C. 1HNMR (DMSO-d6): 6.81 (d, JAB= 7.9Hz, 1Har); 7.03 (q, JBX=
1.9Hz, JAB= 7.9Hz, 1Har); 7.25 (d, JBX= 1.9Hz, 1Har); 11.83 (s, NH).13CNMR (DMSO-d6):101.34 (CH); 109.64 (CH); 123.71 (CH); 135.54 (C); 140.84 (C); 146.14 (C); 165.24 (C=S). HRMS, m/z: 183(M), calcd for C7H5ClN2S: 183.9962, found: 183.996.
5,6-dichloro-2-mercaptobenzimidazole 3e
Yield = 75 %; mp>250°C. 1HNMR (DMSO-d6): 7.79 (s, 1Har); 11.82 (s, NH). 13CNMR (DMSO-d6): 128.60 (CH); 127.25 (C); 135.49 (C); 168.30 (C=S). HRMS, m/z: 217(M), calcd for C7H4N2S: 217.94722, found: 217.9472.
5,6-dimethyl-2-mercaptobenzimidazole 3f
Yield = 80 %; mp>250°C. 1HNMR (DMSO-d6): 2.32(s, CH3); 7.02 (s, 1Har); 12.63 (s, NH). 13CNMR (DMSO-d6): 20.43 (CH3); 111.00 (CH); 131.25 (C); 131.59 (C); 168.00 (C=S). HRMS, m/z: 178(M), calcd for C9H10N3S: 178.05647, found: 178.0564.
Result and Discussion
In this work, we report a novel method for synthesis of 2-mercaptobenzimidazole derivatives. 2- Mercptobenzimidazoles are interesting starting compounds because of their chemical reactivity and biological activities. A mixture of o-phenylenediamines 1(a-g) and N-aminorhodanine in xylene was heated during 8 hours. (Scheme 1). The structure of the products 2(a-g) has been determined by NMR and mass data. 1HNMR spectra showed the presence of the signal of NH and the signal of the C=S was observed in 13CNMR spectra which confirms the structure of the products 2(a-g).
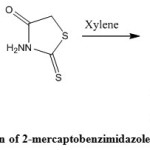 |
Scheme 1: The reaction of 2-mercaptobenzimidazoles synthesis |
A possible mechanism for the formation of the 2-mercaptobenzimidazole (2a-g) is shown in scheme 2. The key step is at the intermediate A, in which the cyclization goes to the C=S group not to C=O group in order to lead to the benzotriazepine product. In the reaction, the only 2-mercaptobenzimidazole product was obtained (Scheme 2)
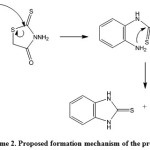 |
Scheme 2: Proposed formation mechanism of the product |
Conclusion
In this work, we developed a novel and improved method for 2-mercaptobenzimidazole derivatives by the condensation of o-phenylenediamines with N-aminorhodanine
Aknowledgements
This work has been supported by the Chouaib Doukkali University
References
- McReynolds, M.D.; Doughtery, J.M.; Hanson, P.R. Chem. Rev. 2004, 104, 2239 – 2258.
CrossRef - Hwang, J.Y.; Gong, Y.D. J. Comb. Chem. 2006, 8, 297 – 303.
CrossRef - Goh, L.Y.; Teo, M.E.; Khoo, S.B.; Leong, W.K.; Vittal, J.J. J. Organomet. Chem. 2002, 664, 161 – 169.
CrossRef - Christensen, C.A.; Meldal, M. J. Comb. Chem. 2007, 9, 79 – 85.
CrossRef - Pae, A.N.; MIN, S. J. ; Roh, E. J.; YANG, H. Y.; Kim, T. H.; PARK, B. G.; Cho, Y. S. U.S. Pat. Appl. Publ. 20140114067, 24 Apr 2014.
- Rivera, A.; Maldonado, M.; Rios-Motta, J. Molecules 2012, 17, 8578 – 8586.
CrossRef - Maske, P.P.; Lokapure, S.G.; Nimbalkar, D.; Disouza, J.I. Pharma Chemica 2012, 4(3), 1283 – 1287.
- Zhivotova, T.S.; Gazaliev, A.M.; Fazylov, S.D.; Aitpaeva, Z.K.; Turdybekov, D.M. Russian Journal of Organic Chemistry 2006, 42(3), 448 – 450.
CrossRef - Valdez, J.; Cedillo, R.; Hernández-Campos, A.; Yépez, L.; Hernández-Luis, F.; Navarrete-Vázquez, G.; Tapia, A.; Cortés, R.; Hernández, M.; Castillo, R. Bioorganic and Medicinal Chemistry Letters 2002, 12(16), 2221 – 2224.
CrossRef - Kidwai, M.; Saxena, S.; Mohan, R. Journal of the Korean Chemical Society 2005, 49(3), 288 – 291.
CrossRef - Li, X. Youji Huaxue 2009, 29(7), 1129 – 1132.
- Kumar, N.D.M.; Dubey, P.K. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry 2012, 51B(11),1619 – 1622.
- Khajavi, M.S.; Hajihadi, M.; Nikpour, F. Journal of Chemical Research, Synopses 1996, 2, 94 – 95.
- Thakuria, H.; Das, G. ARKIVOC 2008, 15, 321 – 328.
- El kihel, A.; Zouitina, S.; Guesmi, S.; Ahbala, M.; Bauchat, P.; Biersack, B. Asian Journal of Chemistry 2016, 28(6), 1267 – 1269.
- El kihel, A.; Ahbala, M.; Ramdane, H.; Nouiri, M.; Bauchat, P. International Journal of Development Research 2016, 6, 7295 -7298.
- El kihel, A.; Essassi, E.M.; Bauchat, P. Arabian Journal of Chemistry 2012, 5, 523 – 526.
CrossRef - El kihel, A.; El ouar, M.; Ahbala, M.; Mouzdahir, A.; Harjane, T.; Knouzi, N. Arabian Journal of Chemistry 2010, 3, 9 – 12.
CrossRef - El kihel, A.; Ahbala, M.; Mouzdahir, A. ; Snader, N. ; Essassi, E.M.; Bauchat, P. ; Danion-Bougot, R. Synthetic Communications 2008, 38, 201 – 205.

This work is licensed under a Creative Commons Attribution 4.0 International License.









