Novel and Efficient Microwave-Assisted Three Component Reaction for the Synthesis Ofoxazine Derivatives
*Preeti Bansal, Nakueshwar Dutt Jasuja and Gajanand Sharma
Suresh Gyan Vihar University, Jagatpura ,Jaipur-302033, India.
Corresponding Author E-mail: bpchemistry08@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320442
Article Received on :
Article Accepted on :
Article Published : 06 Aug 2016
Oxazinederivatives can beprepared with yield upto98% withinafew minutes byanefficientandnovel one potmicrowave- assisted three- componentreaction from 1-naphthol, various anilinesandformalin using montmorilloniteasthecatalyst. The procedureis very simple, efficient and environmentallyfriendlyas itdoesnot useany toxic auxiliary or solvent. The keyadvantagesof this processare high yields, shorterreaction times, and easy work-up andnon – chromatographic method hasbeen used for thepurification of products.
KEYWORDS:solvent free synthesis; multicomponent reaction; oxazine; microwave
Download this article as:| Copy the following to cite this article: Bansal P, Jasuja N. D, Sharma G. Novel and Efficient Microwave-Assisted Three Component Reaction for the Synthesis Ofoxazine Derivatives. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Bansal P, Jasuja N. D, Sharma G. Novel and Efficient Microwave-Assisted Three Component Reaction for the Synthesis Ofoxazine Derivatives. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20064 |
Introduction
Heterocyclic skeletonsserve as ideal scaffold on which pharmacophorecan be appended toyield potent and special drugs. (1) .this is especially true for heterocyclic compound (six member ring), that possess a wide range of interesting biological activities. (2) are corecomponentsof a large number of substances.Oxazinederivatives features prominently in many biologically important natural products (3) and otherbioactive molecules (4-7) the oxazine derivatives have been used as the basic framework for substance of interest in numeroustherapeutic areas such as antifungal(8) , antibacterial, anti-candinaalbicans(9) and kinase inhibitors(10) In chemistry (sustainable)(11) the design and development of sequence allowing highlyselective essence to determine molecularscaffolds when structuraldiversity combined (12) with eco- compatibility. (13)that is great challenges for organic chemists.in asingle operation they build one productfrom three or more reactant molecules with high atom economy (14) and multiple bond forming efficiency of multiple bond forming(15). It is their ability.to reachthis near ideal goal multicomponent reactions are now well established approaches(16). Solvent free reactions are of almost interest from the ecological point of view, and they often advantages, such as reduced reaction time, increased productyields, reduced environmental pollution, simple equipment (lab scale), increased selectivity and low cost compared withreactions carried out in solvents.As an alternative toorganic solvents,chemists should employ other strategies toperformchemical reactions, namely ionic liquid,supercritical fluids, water as solvent andsolvent freeconditions-like inpresence of MW irradiation. This techniquedoes not require solvents and considered“greener” method then the conventional methods. The largescale of applications of microwave chemistry has been increased recently too many aspects of organic synthesis (17-21)Microwave assisted synthesis are aparticularly attractive alternative to synthesis and several thermal conditions since they often proceed much faster and synthesis products with higher yields and higher purity. Although several methods for the preparation of oxazine derivatives have been reported previously (22-25). Some have been focusedon the multicomponent reactions method. This method is advantageous over previous reports due to its short reaction time and solvent free conditions.As per our interest (26-28) to develop better protocol for the synthesis of biologically active heterocyclic compounds, we would like to report the synthesis of oxazine derivatives by the reaction of naphthol, formalin, and aniline in presence of MW irradiation.
Experimental Method
from chemical companies Starting material were purchased and used without purification. All microwave assisted were performed by using microwave synthesizer (CEM corp.) (a discover TM single mode cavity) producing continuous microwave irradiations at 2450 MHz’s. All experiment was conducted under argon. on TLC aluminium roll silica gel 60 F254 (merk) TLC was performed . Melting points weredetermined on a kofler melting point apparatus Melting points were determined. IR spectra were taken on a spectrum one FT-IR spectrometer (Perkin Elmer). UV spectra were measured using a CARY 4E spectrophotometer (Varian). NMR spectrawere recorded on a Varian unity INOVA spectrometer (300/ 75 MHz ) in CDCl3; the1H and C13 chemical shift were referenced to residual solvent signals at δH = 7.25and δC = 77.1 relative to Tetra methyl silane. Mass spectra were recorded on a MAT 90 with 70 ev ionization energy (finnigan MAT)
Experiment
A Mixture of 1a (1.0 m mol), b (2.0 m mol) and c (1.0 m mol) were absorbed on Montmorillonite (76 mg) with methanol and mixed thoroughly and irradiated with Microwaves for a particular time till the reaction are completed., the mixture was allowed to cool to r.t. After completion of the reaction. And washed With EE or TBME (5X5 ml). In vacuo the combined organic extracts were concentrated And by column chromatography the residue was purified on silica gel to yield (4a) Spectral data of compounds:- (4a) yield- 76%, mp.-62-63b , reaction time – 5 min, Anal. Found: C, 82.65%; H, 5.83%; N, 5.48%, Calc.C18H15NO: C, 82.73; H, 5.79; N, 5.36.IR (KBr, νmax/cm-1): 1032 (sym.C-O-C), 1213 (asym. C-O-C); 1H NMR (DMSO-d6, 4, 00 MHz, δ ppm): 4.78 (s, 2H, -Ar-CH2-N-), 5.42 (s, 2H, – O-CH2-N-), 6.81-7.55 (m, 11H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm): 49.2, 79.3, 112.6, 115.2, 117.4, 119.7, 120.6, 124.1, 125.3, 125.3, 126.1, 127.5, 129.1, 132.8, 147.8, 148.7 (4b) yield- 74%, mp.- 300(d), reaction time – 5 min, Anal. Found: C, 78.45; H, 5.90; N, 4.72%, Calc. C19H17NO2: C, 78.33; H, 5.88; N, 4.81. IR (KBr, νmax/cm-1): 1018(sym.C-O-C), 1227(asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 3.61 (s, 3H, OMe), 4.88 (s, 2H, -Ar-CH2-N-), 5.41 (s, 2H, -O-CH2-N-), 6.78-7.81 (m, 10H, Ar -H); 13C NMR (DMSO-d6, 75MHz,,δ ppm): 48.1, 52.1, 80.2, 111.1, 115.5, 117.3, 119.4, 121.2, 124.1, 125.6, 125.8, 126.7, 127.1, 130.2, 132.2, 146.7, 148.5 (4c) Yield- 72%, mp. – 76-770 c, reaction time- 5 min, Anal. Found: C, 78.71; H, 6.28; N, 4.24, Calc. C20H19NO2: C, 78.66; H, 6.72; N, 4.59. IR (KBr, νmax/cm-1): 1027 (sym.C-O-C), 1223 (asym. C-O-C);1H NMR (DMSO-d6, 400 MHz, δ ppm): 1.21 (t, 3H, J = 8 Hz, O-CH2-CH3), 3.91 (q, 2H, J = 8 Hz ,O-CH2-CH3), 4.91 (s, 2H, -Ar-CH2-N-), 5.41 (s, 2H, -O-CH2-N-), 6.81-7.81 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm): 14.5, 48.3, 65.2, 80.5, 112.5, 115.8, 117.3, 119.2, 120.2, 123.2, 124.7, 125.3, 125.5, 126.6, 127.8, 129.9, 132.1, 147.3 (4d) Yield- 76%, mp. – 72-74 c, reaction time – 5 min, Anal. Found: C, 43.58; H, 2.25; N, 2.64, Caic. : C18H12Br3NO, C: 43.41%; H: 2.43%; N: 2.81%; IR (KBr, νmax/cm-1):1015 (sym.C-O-C), 1225 (asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 4.51 (s, 2H, -Ar- CH2-N-), 5.52 (s, 2H, -O-CH2-N-), 6.85-7.91 (m, 8H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm):50.1, 79.2, 106.4, 107.8, 119.4, 124.6, 125.3, 125.8, 126.3, 127.1, 132.5, 133.4, 134.8, 142.7, 147.1, 150.2 (4e) Yield- 70%, mp. – 196-198 c, reaction time – 5 min, Anal. Found: C: 82.71%, H: 6.30%, N: 5.04%., Calc.: C19H17NO, C: 82.88%; H: 6.22%; N: 5.09%; IR (KBr, νmax/cm-1): 1020 (sym.C- O-C), 1233 (asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 2.41 (s, 3H, CH3), 4.91 (s, 2H, -Ar- CH2-N-), 5.61 (s, 2H, -O-CH2- N-), 6.61-7.91 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm):21.1, 49.1, 78.7, 110.3, 115.1, 117.7, 119.5, 120.2, 124.4, 125.4, 125.8, 126.2, 127.3, 129.7, 132.2, 147.7, 148.1 (4f) Yield – 72%, mp.– 200(d), reaction time – 5 min, Anal. Found: C: 78.48%, H: 6.37%, N: 4.67%., Calc: C20H19NO2,C: 78.66%; H: 6.27%; N: 4.59%; IR (KBr, νmax/cm-1): 1022 (sym.C-O-C), 1235 (asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 1.28 (t, 3H, J = 14 Hz, O-CH2-CH3), 3.97 (q, 2H, J = 14 Hz ,O-CH2-CH3), 4.62 (s, 2H, -Ar-CH2-N-), 5.41 (s, 2H, -O-CH2-N- ), 6.18-7.45 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm): 13.8, 48.7, 64.6, 81.4, 111.7, 114.6, 115.7, 117.4, 118.7, 121.1, 122.1, 124.4, 125.5, 125.6, 126.1, 127.5, 128.9, 133.1, 146.2, 149.2 (4g) Yield – 74%, mp. – 118-120 c, reaction time – 5 min, Anal. Found. – C: 77.87%, H: 5.14%, N: 5.15%., Calc.C18H14FNO, C: 77.40%; H: 5.05%; N: 5.01%; IR (KBr, νmax/cm-1): 1027 (sym.C-O-C), 1246 (asym. C-O-C);1H NMR (DMSO-d6, 400 MHz, δ ppm): 4.91 (s, 2H, -Ar-CH2-N-), 5.61 (s, 2H, -O-CH2-N-), 6.81-7.81 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm): 49.1, 78.6, 112.1, 115.6, 116.2, 117.3, 118.1, 122.5, 123.2, 125.2, 125.7, 125.8, 127.6, 128.5, 130.5, 150.1 (4h) Yield – 72%, mp.– 280(d), reaction time – 5 min, Anal. Found – C: 78.21%, H: 5.78%, N: 4.90%., Calc C19H17NO2, C: 78.33 %; H: 5.88 %; N: 4.81 %; IR (KBr, νmax/cm-1): 1028sym.C-O-C), 1212 (asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 3.55 (s, 3H, OMe.), 4.71 (s, 2H, -Ar-CH2-N-), 5.51 (s, 2H, -O-CH2-N-), 6.23-7.64 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75MHz, δ ppm): 49.4, 53.7, 79.5, 112.3, 114.6, 115.9, 117.4, 118.1, 120.8, 124.6, 125.7, 125.8, 126.6, 127.6, 130.2, 133.2, 146.8, 148.8, 151.2 (4i) Yield – 72%, mp. – 86-88 c, reaction time – 5 min, Anal. Found. – C: 82.84%, H: 6.68%, N: 5.14%.,Calc C19H17NO, C: 82.88%; H: 6.22%; N: 5.09%; IR (KBr, νmax/cm-1): 1028 (sym.C-O-C),1232 (asym. C-O-C); 1H NMR (DMSO-d6, 400 MHz, δ ppm): 2.21(q, 3H, CH3), 4.91 (s, 2H, -Ar- CH2-N-), 5.71 (s, 2H, -O-CH2-N-), 6.81- 7.91 (m, 10H, Ar-H); 13C NMR (DMSO-d6, 75 MHz,,δ ppm):20.1, 50.2, 79.2, 113.3, 116.2, 117.3, 119.1, 120.4, 124.6, 125.7, 126.0, 126.7, 127.8,129.6, 130.3, 147.1, 148.2, 149.1, 150.3
Antibacterial Screening
Prepare Muller Hinton agar medium and put into sterile Petriplates.On agarmedium ,200ul of the standard bacterial inoculumswasspread by using sterile cotton swab. In the inoculated agar medium the test impregnated discs were placed. To determinethe sensitivity of each microbial species tested Ciprofloxacin 10ug/ml capacity discs were used as positive references standard. For 24 hours allpetriplateswere incubated
at 37o c. Diameter of inhibition was measured after incubation
Antifungal Screening
Prepared a Sabouraud dextrose agar medium and transferred into sterile petriplates. On agar medium 200 ul of the standardized fungal inoculums were spread by using cotton swab. On the inoculated agar medium the test impregnated discs wereplaced.To determine sensitivity of each microbial species testedClomatrimazole 10 ug/ml was used as positive references standard. For 24 hours all petriplates were incubated at 37 o c. diameter of zone of inhibition was measured After the incubation
Result and Discussing On
our results is present on the microwave – assisted three component synthesis of oxazinederivatives.When amounts of 1(a) aniline, 2(a) 1- naphthol, 3(a) formalin were reacted in the presence ofmontmorillonite in a sealed via under microwave conditions. A focused singlemode microwave reactor for5 minat 1400 c.hasbeenused.Underthese conditions [1, 3] oxazine derivatives 4(a) could be isolated app.In 76% yield.
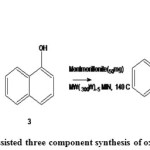 |
Figure 1: microwave assisted three component synthesis of oxazine derivatives Click here to View Figure |
Table 1: Reaction Of Various Aniline With 1- Naphthol And Formalin (1: 1: 2 )
| Entry | Products M.F | R | Reaction time (min) | Mp. (oc) |
| 1234567
8
9 |
C18H15NOC19H17NO2 C20H19NO2 C18H12Br3NOC19H17NOC20H19NO2 C18H14FNO
C19H17NO2
C19H17NO |
H4-OMe4-OEt2,4,6- tri Br4- Me2-OEt4-F
3- OMe
2-Me |
5-105-105-105-105-105-105-10
5-10
5-10 |
62-63b 300(d)76-7772-74196-198200(d)118-120
280(d)
86-88 |
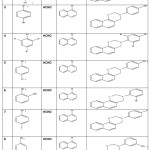 |
Table 2: SYNTHESIS OFOXAZINE DERIVATIVES Click here to View table |
Table 3: Antibacterial Activity Of Compounds (Newly Synthesized Oxazine Derivatives (1A-1I)
| Compound10µg/ml | S.aureus | B.subtillus | E.Coli | P. Aeruginosa |
| 1(a)1(b)1(c)1(d)1(e)1(f)1(g)
1(h)
1(i)
ciprofloxacin |
10151718111516
13
9
19 |
1219211771813
15
8
22 |
817182551921
18
6
26 |
699218817
10
7
22
|
Table 4: Antifungal Activity Of Compounds (Newly Synthesized Oxazine Derivatives (1A-1I)
Compound 10µg/ml |
C. Albicans |
A.niger |
Chrysosporium sp. |
Trichoderma sp. |
| 1a1b1c1d1e1f1g
1h
1i
Clomatrimazole |
91718107177
16
8
19
|
81517129158
13
7
18 |
71419138169
15
9
20 |
61316146148
12
6
18 |
The structure assigned for the reaction products is established from practical data.
Antibacterial Screening
The bacterial inhibition values (mm) are shown in table-3. The antimicrobial activities of compounds S. Aureus, Escherichia Coli, Bacillus Subtillus and P. Aeruginosa were screened. Ciprofloxacin were used as a standard at 100 ug/ml. Compound 1a-1i were screened. S. Aureus for compound 1d was found to be highly active on the other hand for other compounds had low activity with the ciprofloxacin. B. Subtillus shows highly activity for compound 1c, on the other hand for other compoundsshows low activity with the standard ciprofloxacin. E. coli for compound 1d was found to be highly active, on the other hand for other compounds had low activity compared with the standard ciprofloxacin. P. Aeruginosa for compound 1d was found to be highly active, on the other hand for other compounds compound had low activity in comparison the standard ciprofloxacin
Antifungal Screening
The fungal inhibition zone values (mm) are given in table-4. The antifungal activity of compounds C. Albicans, Aspergillus Niger, Chrysosporium sp., Trichoderma sp.Were screened. At a 100 µgmL–1Clomatrimazole were used as a standard.Compound 1a-1i were screened. C. Albicansfor compound 1b and 1f was found to be highly active, on the other hand for compound 1a, 1c, 1d, 1e, 1g, 1h, 1i, had low activity compared with standard Clomatrimazole. Aspergillus Niger for compound 1c was found to be highly active on the other hand for compound 1a, 1b, 1d, 1e, 1f, 1g, 1h, 1i, had low activity compared with standard Clomatrimazole. Chrysosporium sp. For compound 1c was found to be highly active On the other hand for 1a, 1b, 1d, 1e, 1f, 1g, 1h, 1i show low activity with standard Clomatrimazole.Trichoderma sp. for compound 1c was found to be highly active, On the other hand for 1a, 1b, 1d, 1e, 1f, 1g, 1h, 1i show low activity with standard Clomatrimazole.
Conclusion
we have designed an environmentally friendly, green and efficient approach for the synthesis of oxazine derivatives. By solvent free, the microwave assisted three component reactions with yield up to 76% within a few minutes. The method is important due to high conversion, less reaction time, and clean reaction profile, simple experimental and work-up procedure.
Acknowledgment
The author would like to thank the head, department of chemistry, Suresh gyanVihar University, Jaipur (raj.) for the financial support. xz and providing necessary laboratory facilities.
Referance
- Hermakens, P.H.H.; Ottenheijm, H.C.J.; Rees, D.C. Tetrahedron1997, 53, 5643-5678; (b) Gordon, E.M.; Barrett, R.W.;Dower, W.J.; Folder, S.P.A ; Gallop, M.A.J. Med. Chem. 1994, 37, 1385-1401
- Avendano, C.; Menendez, J.C. Medicinal chemistry of Anticancer Drugs; Elsevier; 2008; (b) Doherty, A.M. Annual Reports in medicinal chemistry; Academic Press2000; 35, (c) Patrick, G.L. An introduction to medicinal chemistry; Oxford University; 1995.
- Domling, A., Recent developments in isocyanide based multicomponent reactions in applied Chemistry. Chem.Rev. 2006, 106, 17-89.
CrossRef - Kobayashi, M.J. Kitazawa, M. ;Sotio , T.; Yamamoto, R.; Harada, H. Studies on the synthesis of antiulcer agents. ll. Synthesis and antiulcer activity of cyclic carbamate derivatives. YakugakuZasshi1984, 104, 659-679; chem. Abstr. 1985, 102, 6344 m.
- Testa , E,; Fontanella , L.; Cristiahi, G.; Gallo, G. 5,5 – Disubstituted dihydro-1,3- oxazine -2,4 – diones, research on compounds active on central nervous system XII. J.Org. Chem.1959. 24. 1928-1936.
CrossRef - Vrouenraets, S.M.; Wit, W.F.; Van Tongeren, J.; Lange, J.M. Efavirenz; a review, Expert, Opin, pharmacother.2007. 8. 851-871.
CrossRef - Fauran , C.P.; Douzon, C.; Raynaud, G.; Sergant, M.; Novel derivatives of substituted tetra hydro M-Oxazines, their process of preparation and their therapeutic application., US 3, 821, 215, 1974; Chem. Abstr.1974, 82, 125412
- Fringuelli, R.; Pietrella, D.; Schiaffella, F.; Guarra, I.A.; Perito, S; Bistoni, F.; Vecchiarelli, A.; Biorg. Med. Chem. 2002, 10, 1681-1686
CrossRef - Macchiarulo, A.; Costantino, G.; Fringuelli, f.; Vecchiarelli, A.; Schiaffella, F.; Fringuelli, R. Bioorg. Med. Chem. 2002, 10, 3415-3423
CrossRef - Adans, N.D.; Darcy, M.G.; Dhanek, D.; Duffy, K.J.; Fitch, D.M.; Knight, S.D.; Newlander, K.A.; A.N.Int. Patent. Wo2006, 113432, 2006; Chem. Abstr.2006, 145, 438652
- Special issue in Green chemistry, See: Chem. Rev., 2007,107, 2167
- Schreiber,S.L.;Nature,2009, 457, 153.
CrossRef - We define eco-compatible as both economically and ecologically compatible
- Trost,B.M. ;Acc. Chem. Res.; 2002, 35, 695
CrossRef - Wender, P.A. ;Verma,U.A. ;. Paxton,T.J ;and. Pillow,T.H;Acc. Chem. Res.,2008, 41, 40
CrossRef - Zhu,ed. J++ and Bienayme, H.; Wiley –VCH, Multicomponent reactions, Weinheim,2005
- Wender,P.A.; Handy,S.T.; And. Wright, D.L; Chem. Ind., 1997, 765
- De Simore, J.M.; Practical approaches to green solvents. Science, 2002, 297, 799-803
CrossRef - Sharma J, Chin B, Huibers PDT, et al. Solvent replacement for green processing.Environ. Health perspect.,1998,106 (Suppl.1): 253-271,.
- Roberts B A, Strauss CR. Towards rapid, “green” predictable microwave-assisted synthesis. Review. Acc. Chem. Res.,2005, 38, 653-66
CrossRef - Larhed M, Moberg C, Hallberg A. Microwave accelerated homogeneous catalysis in Organic chemistry.Acc. chem. Res.,2002, 35, 717-727,
CrossRef - Caddick S.;Microwave assisted organic reactions. Tetrahedron;1995,51, 10403-10432,
CrossRef - Agag, T.; Preparation and properties of some thermosets derived from allyl-functional naphtooxazines. J. App. Poly. Sci. 2006, 100, 3769-3777
CrossRef - Burke W.J.; Murdock, K.C.; Ec, G.;Condensation of hydroxy aromatic compounds with formaldehyde and primary aromatic amines. J. Am. Chem. Soc. 1954, 76, 1677-1679
CrossRef - Mathew, B.P.; Nath, M.;One pot three component synthesis of dihydrobenzo and naphtho [e] – 1, 3 –oxazines in water. J. Heterocyclic Chem. 2009, 46, 1003-1006
CrossRef - Sapkal, S.B.; Shelke, K.F.; Shingate, B.B.; Shingare, M.S. ;Nickel nanoparticle catalyzed facile and efficient one pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent free conditions. Tetrahedron Lett.2009, 50, 1754-1756.
CrossRef - Sonar, S.S ; Kategaonkar, A.H.; Ware, M.N.; Grill, C.H.; Shingate, B.B.; Shingare, M.S.; Ammonium metavanadate: an effective catalyst for synthesis of α- hydroxyphosphonates. Arkivoc2009, ii, 138-148.
- Sonar, S.S.; Sadaphal, S.A.; Kategaonkar, A.H.; Pokalwar, R.U.; Shingate, B.B.; Shingare, M.S.;Alum Catalysed Simple and efficient synthesis of bis (indolyl) methanes by Ultrasound Approach. Bull. Korean Chem. Soc.2009, 30, 825-828.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









