Novel and Efficient Method for The Synthesis of 4-Chloro-5, 6-Dihydro Pyran Derivatives using Lewis Acidic Chloroaluminate Ionic Liquids
N. Maruthi Raju2, K. Rajasekhar1*, J. Moses Babu2 and B. Venkateswara Rao3
1Ragas Pharmaceuticals Private Limited (OPC), IDA Cherlapally, Hyderabad, 500051, India.
2Custom Pharmaceutical Services, Dr Reddy's Laboratories Limited, Bollaram Road, Miyapur, Hyderabad, 500049, India.
3Department of Organic Chemistry, Foods, Drugs and Water, Andhra University, Visakhapatnam, 530003, India.
Corresponding Author E-mail: koorella.rajasekhar@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320459
The reaction of aldehydes/ketones with homopropargylic alcohols in the presence of 1-n-Butyl-3-methylimidazolium chloroaluminate [bmim]Cl·AlCl3 (N = 0.56-0.67) ionic liquid generates the 4-chloro-5,6-dihydro-2H-pyran derivatives in excellent yield and in short reaction times.
KEYWORDS:1-n-Butyl-3-methylimidazolium chloroaluminate; aldehydes; ketones; homopropargylic alcohols; 4-chloro-5,6-dihydro-2H-pyran derivatives
Download this article as:| Copy the following to cite this article: Raju N. M, Rajasekhar K, Babu J. M, Rao B. V. Novel and Efficient Method for The Synthesis of 4-Chloro-5, 6-Dihydro Pyran Derivatives using Lewis Acidic Chloroaluminate Ionic Liquids. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Raju N. M, Rajasekhar K, Babu J. M, Rao B. V. Novel and Efficient Method for The Synthesis of 4-Chloro-5, 6-Dihydro Pyran Derivatives using Lewis Acidic Chloroaluminate Ionic Liquids. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=18900 |
Introduction
Substituted dihydropyrans are the key intermediates for the synthesis of many natural products1.Many natural products like Swinholides2a, Laulimalides2b, Ambruticins2c and Jerangolids2d contains dihydropyran skeleton. Moreover, the olefin function is having synthetic value for further functionalization in obtaining polysubstituted tetrahydropyrans3. The coupling of alkynes to aldehydes is an important transformation in organic synthesis4. The direct synthesis of dihydropyrans by the coupling of alkynes to aldehydes provides a useful synthetic method for the synthesis of dihydropyrans. Although other methods were reported5, Consequent methods that successfully minimize the use of toxic and volatile organic solvents are the focus of much attention. In this respect, ionic liquids are attracting growing interest as alternative reaction media for various chemical and biotransformations6. In particular, choloroaluminate ionic liquids are having Lewis acidity, which can be varied over a wide range, and their intrinsic ability to solvate a variety of substances. These ionic liquids are easily prepared from AlCl3 and 1-butyl-3-methylimidazolium chloride. These chloroaluminate ionic liquids have the advantage of being liquid at room temperature over a considerable composition range of apparent mol fraction of AlCl3 (N = 0.30-0.67) and also have negligible vapour pressures, making them useful alternatives to conventional molecular organic solvents for various synthetically useful transformations7. Furthermore, chloroaluminate ionic liquids play dual roles both as Lewis acid catalyst and as solvent.
Results and Discussion
In view of the emerging importance of the use of Ionic liquids as cost-effective and environmentally benign catalysts, we herein describe a simple and efficient protocol for the cyclization reactions of aldehydes and homopropargylic alcohols to produce dihydropyrans using 1–n-Butyl-3-methylimidazolium chloroaluminate [bmim]Cl·AlCl3 (N = 0.56-0.67) ionic liquid under mild reaction conditions (Scheme I).
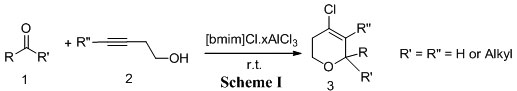
For instance treatment of benzaldehyde with 3-butyn-1-ol in [bmim]Cl·AlCl3 ionic liquid afforded dihydropyran in 70% yield. The reaction is very clean and complete within 30 sec. at room temperature. In a similar manner, various aldehydes and ketones underwent smooth cyclization reaction with homopropargylic alcohols to give the corresponding dihydropyran derivatives in high yields. In all cases, the reactions proceeded readily at room temperature with high efficiency. The reaction worked well both with aromatic, aliphatic aldehydes and ketones. When symmetrical ketones like cyclohexanone and 3-pentanone reacted with 2c and 2d the formation of single product was observed. But when applied to aldehydes the formation of a mixture of the isomers were observed by TLC and 1HNMR spectrum. This is due to the formation of the diastereomers in the later case (Scheme II).

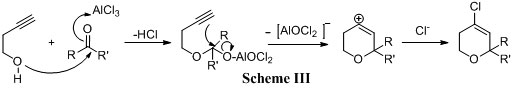
The mechanism for the formation of dihydropyrans can be explained by the attack of homopropargylic alcohol and cyclised to the dihydropyran carbenium ion which is further attacked by the chloride nucleophile to form the 4-Chloro dihydropran derivative (Scheme III).
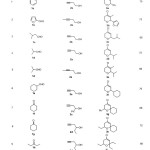 |
Table 1 Click here to View table |
Conclusion
In summary, we have described a green protocol for the preparation of dihydropyran derivatives through cyclization reaction of aldehydes/ketones with homopropargylic alcohols using 1–n-Butyl-3-methylimidazolium chloroaluminate ionic liquidsystem. The attractive features of this process are the mild reaction conditions, eco-friendly reagent, short reaction times and cleaner reactions with good yields, which makes it a useful process for the synthesis of dihydropyran core structure.
Experimental Section
Chloro Aluminate Ionic liquids were prepared as described previously8.
General Procedure
To a mixture of benzaldehyde (500 mg, 4.71 mmol) and 3-butyn-1-ol (330 mg, 4.71 mmol) was added 1–n-Butyl-3-methylimidazolium chloroaluminate (2 mL) at room temperature. The mixture was stirred for 30 sec. and the reaction mass was quenched with icecold water and extracted with diethyl ether (10-15 mL). The combined organic layers were dried over anhydrous Na2SO4, concentrated in vacuo and purified by column chromatography on silica gel (Merck, 60-120 mesh, ethyl acetate/hexane, 2.0-8.0) to afford dihydropyran 3a. The products were characterized by IR, NMR and mass spectroscopy. All the products 3b-i were prepared by the same procedure.
Acknowledgements
NMR thanks Dr Reddy’s Laboratories for permitting the research work.
References
- Oishi T., Ohtsuka Y., Studies in Natural Products Synthesis, edited by Atta-ur-Rahman, (Elsevier, Amsterdam), 3, 1989, 73; (b) Yet L, Chem Rev, 100, 2000, 2963.
- Hayakawa H & Miyashita M, Tetrahedron Lett, 41, 2000, 707; (b) Mulzer J & Hanbauer M, Tetrahedron Lett, 2000, 41, 33; Connor D T, Greenough R C & Strandtmann M, J. Org. Chem, 42, 1977, 3664; (d) Gerth K, Washausen P, Hoftle G, Irschik H & Reichenbach H, J Antibiot, 49, 1996, 71.
- Boivin T L B, Tetrahedron, 43, 1987, 3309; (b) Coppi L, Ricci A & Taddei M J Org Chem, 53, 1988, 913 (c) Li C J & Zhang W C, Tetrahedron, 56, 2000, 2403 (d) Schmidt B & Westhus M, Tetrahedron, 56, 2000, 2421.
- Yadav J. S, Reddy B V S & Vishnumurthy P, Tetrahedron Lett, 46, 2005, 1311; (b) Frantz D E, Fassler R, Carreira E M, J Am Chem Soc, 122, 2000, 1806; (c) Anand N K, Carreira E M, J Am Chem Soc, 123, 2001, 9687; (d) Tzalis D & Knochel P, Angew Chem, 38, 1999, 1463 (e) Miranda P O, Diaz D D, Padron J I, Bermejo J & Martin V S, Org Lett, 5, 2003, 1979.
- Miranda P O, Diaz D D, Padron J I, Bermejo J & Martin V S, Org Lett, 5, 2003, 1979
- Sheldon R, Chem Commun 2001, 2399; (b) Welton T Chem Rev, 99, 1999, 2071; (c) Wasserscheid P & Keim W, Angew Chem Int Ed, 39, 2000, 3772.
- Namboodari V V & Verma R S Chem Commun 2002, 342; (b) Potdar M K, Mohlile S S & Salunkhe M M, Tetrahedron Lett, 42, 2001, 9285; (c) Ren R X & Wu J X, Org Lett 3, 2001, 3727.
- Wilkes J S, Levisky J A, Wilson R A & Hussey C L, Inorg Chem, 21, 1982, 1263.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









