Modeling of Competitive Ultrasonic Assisted Removal of The Crystal Violet and Aura Mineo using Mwcnts Functionalized by N-(3-Nitrobenzylidene)-Nˊ-Trimethoxysilylpropyl-Ethane-1,2-Diamine:Equilibrium, Kinetics and Thermodynamic Study
Farveh Raoufi, Hossein Aghaee and Majid Monajjemi*
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran P.O.Box14515-755, Tehran, Iran.
Corresponding Author E-mail: monajjemi@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320412
Article Received on :
Article Accepted on :
Article Published : 19 Jul 2016
In this study, adsorbent was synthesized by covalently anchoring N-(3-nitro-benzylidene)-N-trimethoxysilylpropyl-ethane-1, 2-diamine onto multi-walled carbon nanotubes (NBATSPED-MWCNTs). This novel material was characterized by different techniques such as XRD, SEMand FT-IR.Subsequently, itwasusedfortheultrasound-assisted removalofAura mine O(AO) and Crystal violet (CV) from aqueous solutions was investigated. The dependency of removal percentages to variables such as pH, initial dyes concentration,adsorbent dosage, sonication time on the removal percentages ofAO and CV were simultaneously investigated by central composite design (CCD) under response surface methodology (RSM). It was shown that the adsorption of AO and CV follows the pseudo-second-order rate equation, while the Langmuir model explains equilibrium data. Isotherms had also been used to obtain the thermodynamic parameters such as free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) of adsorption. The negative value of ΔG° indicates the feasibility and spontaneity of the adsorption process. The positive ΔH° suggests the endothermic nature of the adsorption. The positive values of ΔS0 reflect the affinity of multi-walled carbon nanotubes functionalized towards CV and AO. A small amount of the adsorbent was able to remove more than 99.20% of both dyes rapidly with high adsorption capacity in binary-component system (69.36 mgg-1 and 120.65mg g-1for AO and CV respectively).
KEYWORDS:multi-walled carbon nanotubes; Response surface methodology; Ultrasound-assisted dye removal; Crystal Violet; Aura mine O
Download this article as:| Copy the following to cite this article: Raoufi F, Aghaee H, Monajjemi M. Modeling of Competitive Ultrasonic Assisted Removal of The Crystal Violet and Aura Mineo using Mwcnts Functionalized by N-(3-Nitrobenzylidene)-Nˊ-Trimethoxysilylpropyl-Ethane-1,2-Diamine:Equilibrium, Kinetics and Thermodynamic Study. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Raoufi F, Aghaee H, Monajjemi M. Modeling of Competitive Ultrasonic Assisted Removal of The Crystal Violet and Aura Mineo using Mwcnts Functionalized by N-(3-Nitrobenzylidene)-Nˊ-Trimethoxysilylpropyl-Ethane-1,2-Diamine:Equilibrium, Kinetics and Thermodynamic Study. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19151 |
Introduction
The colored dye effluents are considered to be highly toxic to the aquatic biota and affect the symbiotic process by disturbing the natural equilibrium by reducing photosynthetic activity and primary production due to the colorization of the water1, 2. Synthetic colors are widely used in food industries, while some of them may generate hazards andproblem to human health at excess level3-5. Colors and dyes hinder from light penetration and lead to production of carcinogenic and mutagenesis hazards6. These problems make emergency to design and develop new protocol to treat them and achieve a safe and clean media7. Auramine O (AO) and its hydrochloride salt are used in the coloring of paper, textiles and leather; also as food dye8.International Agency for Research on Cancer included AO is sufficient evidence of carcinogenicity in experimental animals due to its bio-transformation to reactive species in target organs of both rats and humans9. AO, and its hydrochloride salts are used for coloring paper, textiles and leather10. International Agency for Research on Cancer (IARC) expressed more evidence of carcinogenicity ability of AOamong chemicals related to its bio-transformation to reactive species in target organs of rats and humans11, 12.
As a typical cationic dye, crystal violet (CV) belongs to the triphenylmethane group, which is widely applied in coloring paper, temporary hair colorant, and dyeing cottons and wools. CV may harm the body via inhalation, ingestion and skin contact. It has also been found to cause cancer and severe eye irritation to human beings13-15. Adsorption is one of the best and simple techniques for the removal of toxic and noxious impurities in comparison to other conventional protocols like chemical coagulation, ion exchange, electrolysis, biological treatments is related to advantages viz. lower waste, higher efficiency and simple and mild operational conditions16-18. Adsorption techniques also have more efficiency in the removal of pollutants which are highly stable in biological degradation process through economically feasible mild pathways19-21. Of these methods, nanomaterial’s based adsorbents is highly recommended for dyes pollutants removal22-26. The best figures of merit in multi component dyes systems removal are based on development of novel method that permits their accurate simultaneous determination in mixtures. The encounter difficulties are serious peaks overlapping that subsequently impossible their direct determination in mixture using general equation like Beer–Lambert26-28.
Conventional and classical optimization protocol (one at a time) failed to give useful knowledge about the interaction between variables while requiring intensive labor and consuming long time because of requiring high number of experiments. Central composite design (CCD) under response surface methodology (RSM) can efficiently be applied for handling both variables involved and responses without suffering above-mentioned draw backs29-31.This approach enables researchers to make a suitable predicative model to apply for several factors even in the presence of complex interactions to describe responses and find the optimal conditions with less time elapsed32-35. In this work, MWCNT was modified with Schiff base-like structure following its reaction with N-(3-nitro-benzylidene)-N0-trimethoxysilylpropyl-ethane-1, 2-diamine (NBATSPED-MWCNT).A novel adsorbent followed by the characterization using different techniques such as Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and scanning electron microscopy (SEM). This adsorbent was used for the ultrasound-assisted removal of the CV and AO from aqueous solutions. The influences of variables such as sonication time, amount of adsorbent, initial CV and AO as well as their possible interactions were investigated and successfully optimized by central composite design (CCD) under response surface methodology (RSM). The adsorption kinetics and isotherms were also studied
Experimental
Materials and Instruments
Auramine and Crystal violet are fully described in Table1.They were supplied from Merck (Darmstadt, Germany). The stock solution (100mgL-1) of each dye was achieved following dissolution of 10 mg of which in100 mL double distilled water, separately and suitable dilute on was performed to obtain working solution with desiredconcentration, N-(3-(trimethoxysilyl)-propyl)ethylene diamine(TSPED) and 2-nitrobenzaldehyde (NBA) were purchased from Sigma-Aldrich. Multiwall carbon nanotubes (MWCNTs) were supplied from Riedel-de Hean (Hannover, Germany), and other reagents were used in analytical grade from Merck Company. The pH was adjusted and measured using pH/Ion meter model 686 (Metronome, Switzerland, Swiss). AO and CV concentrations were determined using UV–Vis spectrophotometer model V-530 (Jasco, Japan) at wave-lengths of 434 nm and 588 nm, respectively.
The ultrasonic bath with heating system (Techno-GAZ SPA Ultrasonic System, Italy) was used at frequency 60Hz and power 130W. The morphology of the NBATSPED-MWCNT was studied by scanning electron microscopy (SEM; HitachiS-4160). The FTIR spectra of compounds were recorded on aJASCO-680 instrument in the range of 400-4000 cm−1 using KBr pellet with ratio 1: 100 for samples to KBr. X-ray diffraction (XRD) pattern was recorded by an automated Philips X’PertX-ray diffractometer with CuKα radiation (40 kV and 30 mA) for 2θ values .The softwareDesign-Expert® software Version. 7.0was used for experimental design analysis and their subsequent regression analysis.
Preparation of NBATSPED-MWCNT
At first step, trimethoxysilylpropylethylenediamine supported on MWCNT (NH2-MWCNT) was synthesized by the reaction of 1.8 mL N-(3-(trimethoxysilyl)-propyl)ethylene diamine and 0.1 g MWCNT in 20 mL of dichloromethane under reflux at 40 °Cin the oil bath for 24. Then, the obtained solid was filtered, rinsed sequentially with ethanol and dried in an oven at 50 °C.Then, 0.9 g of 2-nitrobenzaldehyde was added to the resulting substance in 20 mL of methanol and refluxed at 60 °Cin oil bath for 24 h. The product was filtered, washed with 50 mL of ethanol, distilled water and then dried in oven for 10 h for50 °C. In this way, N-(3-nitro-benzylidene)-N0-trimethoxysilylpropyl-ethane-1, 2-diamine supported on MWCNT (NBATSPED-MWCNT) was obtained as a new adsorbent. The steps for the synthesis of the adsorbent are presented in Scheme 1.
Ultrasound-assisted multi-component adsorption of AO and CV on to NBATSPED-MWCNT
A batch process using NBATSPED-MWCNT in presence of ultrasound was applied for binary adsorption of AO and CV, while all experiments were under taken in a cylindrical glass vessel by adding 0.032g of adsorbent to 50 mL of AO and CVat pH 5.0 as optimum value. The vessel was immersed in an ultrasonic bath for 4 min at room temperature and subsequently the solutions were centrifuged. Then, non-adsorbed dye contents were determined by using UV–Vis spectrophotometer set at wavelengths 434 and 584 nm for AOand CV, respectively.
Measurements of dye uptake
The dye concentrations were determined from calibration curve obtained at maximum wavelength over working concentration range. The efficiency of dyes removal was determined at different experimental conditions optimized using the CCD method. The experiments were also performed in the initial dye concentration range of 5-25mg L-1to obtains adsorption isotherms. The removal percentage of each dye was calculated using the following equation25:
![]()
Where, Ce (mg L-1) and Ct (mg L-1)is the concentration of target at initial and after time t respectively36.
![]()
where C0 (mg L-1)and Ce (mg L-1)are the initial dye concentration and equilibrium dye concentration in aqueous solution, respectively, V (L) is the solution volume and W (g) is the adsorbent mass.
Central Composite Design (CCD)
Response Surface Methodology is a statistical method that uses experimental data obtained from specified experimental design to model and optimize any process in which response of interest is influenced by several variables37,38. Primarily, this optimization is done by following three major steps, viz., performing the statistically designed experiments, estimating the coefficients in a mathematical model, and predicting their responses followed by examining the adequacy of the model39. RSM helps to enumerate the relationships between output variables called responses(y) and input variables called factors (Xi S) 40.
![]()
CCD under RSM is suitable for fitting a quadratic surface to optimize the effective parameters and to investigate the contribution of variables and the interaction with minimum number of experiments41. Generally, the CCD consists of 2nfactorial runs with 2n axial runs and nCcentral runs where n is the number of variables( n=5 in this work)42, while the replication of central point gives a sense on numerical values of experimental errors and the data reproducibility. Thus, conducting 32 experiments permits the researchers for full study of all regions. Each experimental run was analyzed and the response was correlated with five input factors by using the following quadratic polynomial equation29:
![]()
Where y is the predicted response (removal percentage); XiSare the independent variables AOandCV concentration, pH, amount of adsorbent and sonication time) that are known for each experimental run. The parameter β0is the model constantβiis the linear coefficient; βiiare the quadratic coefficients and βijare the cross-product coefficients and εisthe residual term. The regression analysis is used to fit the equations for both responses to the experimental data and to estimate the statistical significance of the equation using the Design-Expert® software Version. 7.0software.
Obtain an optimal response. The first or second-order polynomial equations obtained according to the experimental responses as well as their subsequent fitting and the analysis of variance (ANOVA) help to estimate the main contribution of variables involved and their interaction43. The plot of three-dimensional graph leads to the generation of surface response applied for the prediction of best operating conditions according to P-values and F-values. In this work, the dependence of dyes removal percentages as responses on five factors such as the initial AO concentration, initial CV concentration, pH effect, amount of adsorbent and sonication time at five levels (Table2) was analyzed using the software Design-Expert® software according to 32 runs.
Desirability function (DF)
Desirability function (DF) creates a function for each individual response dileading to final output of global function (D), maximum value of which supports the achievement of optimum value44. The principle and application of desirability function for the best predication of real behavior of adsorption system was pointed out previously35. The desirability profiles indicate the predicted levels of variables, which produce the most desirable responses.
Results and Discussion
Characterization of adsorbent
TheFTIR spectrum ofNBATSPED-MWCNTs (Fig 1), shows absorption peak at 1715 cm-1 corresponding to the stretching vibration of carbonyl groups. The broad peaks at 1086 cm_1 could be assigned to C–O stretching fromphenolic, alcoholic, etheric groups and to C–C bonds. The new peak appearing at≈3400 cm-1 corresponds to OH stretching. This peak can be assigned to the hydroxyl group of moisture, or carboxylic groups. The aromatic C=C stretch is observed at≈1459 cm-1. The FT-IR spectrum of NH2-MWCNT displays a new peak as a weak shoulderat2931 cm-1, which corresponds to the stretching vibrations of C–H bonds in propyl group. After the addition of 2-nitrobenzaldehyde, the new peak appeared at 1635 cm-1 is related to C=N which indicates successful synthesis ofNBATSPED. Following the addition of NBA (Fig. 1), two peaks at 1324 and 1585 cm-1 are attributed to –NO2groups which confirm again the success of this step[45]the morphological features of the samples studied by SEMare shown in(Fig. 2).After the surface modification with NBATSPED, the NBATSPED-functionalized MWCNTs became rough, larger and bundled46. The XRD pattern of the NBATSPED-MWCNTs (Fig.3) represents a peak at 25.961 (002) corresponding to the interlayer spacing of the nanotube. The peaks at 43.05(100), 53.92(004)and 78.5(100) correspond to diffractions and reflections from the carbon atoms47 As seen, thehighly crystalline nature of theMWCNTs after functionalizing with NBATSPEDis confirmed, while the high intensity of peak at 53.49 (004) shows that there has been a small amount of material in amorphous state. Theobserved XRD pattern indicates that the prepared NBATSPEDMWCNTis well-synthesized.
Analysis of Central Composite Design
As it is seen from CCD(Table2), the effects of five dependent variables AO concentration (X1), CV concentration (X2), pH (X3), amount of adsorbent (X4) and time of sonication (X5) were investigated. 32 experiments and their corresponding responses are presented in Table 2. Analysis (ANOVA) was performed for the removalPercentageof variance of each dye by using Design-Expert® software Version. 7.0.(Table 2) the quality of the polynomial model equation was judged statistically by the coefficient of determination and its statistical significance was determined by F-test. P-values less than 0.05 are generally considered as a criterion for distinguishing statistically significant variables. However, Bonferroni test was applied to reduce alpha value to 0.01 which results in reducing the number of significant variables. As mentioned above, a P-value less than 0.01 (Bonferroni adjusted) indicates the statistical significance of each factor at 95% confidence level. Thus, the following predictive models describing the removal percentages of (AO) and (CV) dyes were obtained in terms of significant variables which satisfy the Bonferroni limit.
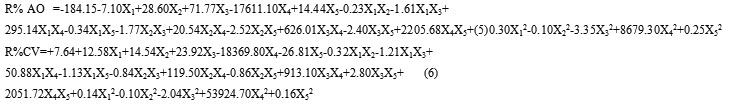
The plot of experimental R% values versus predicted ones indicated a good fit for both AO and CV (Fig.4). From ANOVA for the R% AO and R% CV, the P-values for the lack of fits corresponding to AO and CV were obtained to be 0.16 and 0.33, respectively which prove the applicability of the predictive models. For both models, and adjusted were close to 1 confirming the goodness of the fit13. From Eq. (5), it is seen that the pH and its interaction with both AO and CV concentrations are significant which negatively affect R% AO. Another significant variable that positively affects R%AO is amount of adsorbent. The trend in Eq. (6) corresponding to R%AO is fairly similar to that of AO. However, CV concentration significantly affects the R%CV negatively in linear and quadratic ways. Furthermore, the interaction of the pH with amount of adsorbent played significant role in R% AO and R% CV.
Response surface plots
The 3D RSM surfaces corresponding to R%AO and R%CVwere depicted and considered to optimize the significant factors and to give useful information about the possible interaction of variables. As also seen from Eqs.(5, 6), the effects of significant interaction terms on the curvature of the surfaces are observed as expected (Fig.5and 6). Fig (5a) shows the interaction of adsorbent dosage within initial dye concentration and their relation with removal percentage. It was seen that the removal percentage of dye decrease at higher initial dye concentration. This was also because of increase in the ratio of dye molecules to available adsorption surface area.
Fig (6a) shows the three dimensional response surface of the combined effect of weight and initial concentration on percentage removal with increase in initial concentration, removal decreases when weight of NBATSPED – MWCNT is kept constant. This is because at constant weight of NBATSPED – MWCNT when initial concentration of dye is increasing adsorbent gets saturated and it cannot adsorb further. Whereas with increase in weight of NBATSPED – MWCNT percent removal increases monotonously. The reason is that when weight ofNBATSPED – MWCNT is more, then the surface area available is also high, that increases the percentage removal.
The response surfaces plots Fig. 6b demonstrated the removal percentage dependency as a function of adsorbent dosage and contact time, the removal percentage increased with enhance in adsorbent dosage due to its high specific surface area and small particle size. At higher value probably due to increase in surface area and availability of more active adsorption sites the rate of adsorption significantly increased. At lower amount of NBATSPED – MWCNT, due to insufficiency of reactive sites and lower ratio of dye molecule to vacant site removal percentage significantly decreased.
Optimization of CCD by DF for extraction procedure
TheprofilefordesirableoptionwithpredictedvaluesintheDesign-Expert software wasusedfortheoptimizationofthe process (Fig.7).Thedesirabilityintherange of 0.0 (undesirable) to 1.0 (very desirable) was used to obtain a global function (D) that is the base of optimization. Based on these calculations and desirability score of 1.0,maximum removal (100 and 97.00%for AO and CV, respectively)was obtained at optimum conditions as: 4 min for sonication time, 0.032g for adsorbent mass, 25 mgL-1 for AO and 15 mgL-1 for CV at pH 5.0. Five solutions with different amounts of ideal conditions were used to predict the optimum conditions for AO and CV dyes adsorption on to NBATSPED-MWCNT. The validity of the predicated responses at the optimized conditions was checked by performing duplicate experiments at similar conditions with RSD lower than 2% and removal percentage more than 97.5% in good agreement with the predicted value. This strong agreement proves that the predictive model found based on RSM is well applicable for describing the removal percentages of dyes studied against the variables involved.
Adsorption equilibrium study
Adsorption equilibrium isotherm is designed based on mathematical relation of the amount of adsorbed target per gram of adsorbent (qe(mgg-1)) to the equilibrium non-adsorbed amount of dye in solution (Ce(mgL-1) at fixed temperature48,49. Isotherm studies are divided to well-known models such as Langmuir, Freundlich, Temkin and Dubinin–Radushkevich based on well-known conditions. The Langmuir model is the most frequently employed model given by following equation50:
![]()
WhereCe, QmandKL are the concentration of adsorbate at equilibrium (mgL-1), maximum monolayer adsorption capacity (mgg-1) and Langmuir constant (Lmg-1), respectively.Ce/qewas plotted against Ce where parameters such asQm, KL, and R2 were calculated based on the slope and intercept of such lines and displayed in Table4. The values of Ka (the Langmuir adsorption constant (Lmg-1)) and Qm(theoretical maximum adsorption capacity (mgg-1)) were obtained from the intercept and slope of the plot of Ce/qever.Ce, respectively. The applicability of Langmuir modelfor the interpretation of the experimental data over the whole concentration range is proven from high correlation coefficient at all adsorbent masses. The increase in the amount of adsorbent leads to significant enhancement in the actual amount of adsorbed dye. The parameters of Freundlich isotherm model such as KF and the capacity of the adsorption were calculated from the intercept and slope of the linear plot of lnqeversuslnCe, respectively51. The heat of the adsorption and the adsorbent–adsorbate interaction were evaluated by using Temkin isotherm model52. In this model, B is the Temkinconstant related to heat of the adsorption (Jmol-1), T is the absolute temperature(K), R is the universal gas constant(8.314Jmol-1 K-1) and K is the equilibrium binding constant(Lmg-1). D–R model was applied to estimate the porosity apparent free energy and the characteristic of adsorption53. In this model B (mol2/Kj2) is a constant related to the adsorption energy, QS(mgg-1) is the theoretical saturation capacity and E is the Polanyi potential. The slope of the plot of lnqe versusε2gives B and its intercept yields the QSvalue. The linear fit between the plot of Ce/qeversusCe and calculated correlation coefficient (R2) for Langmuir isotherm model shows that the dye removal isotherm can be better represented by Langmuir model (Table4). This confirms that the adsorption of AO and CVdyes takes place at specific homogeneous sites as a monolayer on to the NBATSPED-MWCNT surface.
Kinetic study
Adsorption of a solute by a solid in aqueous solution through complex stages54 is strongly influenced by several parameters related to the state of the solid (generally with very heterogeneous reactive surface) and to physico- chemical conditions under which the adsorption occurred. The rate of dyes adsorption onto adsorbent was fitted to traditional models like, pseudo-first, second-order, intra-particle and Elovich models. The Lagergren pseudo-first order modeled scribed the adsorption kinetic data55. The Lagergren is commonly expressed as follows:
![]()
Where, qt and dqt(mgg-1) are the adsorption capacities at equilibrium and at time t, respectively. k1 is the rate constant of the pseudo-first-order adsorption(Lmin-1). The log (qe−qt) versus t was plotted and the values of k1 and qewere determined by using the slope and intercept of the line, respectively56.
![]()
The fact that the intercept is not equal toqe imply that there action is unlikely to follow the first-order57. The relationship between initial solute concentration and rate of adsorption is linear when pore diffusion limits the adsorption process. Therefore, it is necessary to fit experimental data to another model (Table5) such as pseudo-second order model58, based on the following equation:
![]()
Eq.(11) is integrated over the interval 0 to t for t and 0 to qtforqeto give
![]()
As mentioned above, the plot oflog (qe−qt) versus t does not show good results for entire sorption period, while the plot of t/qtversus t shows a straight line. The values of k2 and equilibrium adsorption capacity qewere calculated from the intercept and slope of the plot of t/q versus t (Table5). The calculated qevalues at different working conditions like various initial dyes concentrations and/or adsorbent masses were close to the experimental data and higher R2 values corresponding to this model confirm its more suitability for the explanation of experimental data. This indicates that the pseudo-second-order kinetic model applies better for the adsorption of AO and CV system for the entire sorption period the intraparticle diffusion equation is given as59:
![]()
Where kdifis the intraparticle diffusion rate constant (mg/(g. min1/2)) and C shows the boundary layer thickness. The linear form of Elovich model is generally expressed as60:
![]()
The kinetic data from pseudo-first and pseudo-second-order adsorption kinetic models as well as the intraparticle diffusion and Elovich model are given in Table5. The linear plots oft/qt versus t indicated a good agreement between the experimental and calculatedqe values for different initial dyes concentrations. Furthermore, the correlation coefficients of the pseudo-second-order kinetic model (R2>0.99) were greater than that of the pseudo-first-order model (R2<0.95). As a result, the adsorption fits to the pseudo-second-order better than the pseudo-first-order kinetic model.
Theoretical approach
In this work we have modeled some parts of our systems based on works 61-197 and we will start a theoretical calculation for any further research in this area.
Thermodynamic studies
The thermodynamic parameters, the values of enthalpy (ΔH0), and entropy (ΔS0), and Gibbs free energy (ΔG0), of the sorption are useful in defining whether the sorption reaction is endothermic or exothermic and spontaneity of the adsorption process55the adsorption standard free energy changes (ΔG0) can be calculated according to198:
![]()
Where, R (8.314 Jmol−1 K−1) is the ideal gas constant, and T (K) is the temperature. The negative values at different temperatures were due to the fact that the adsorption process is spontaneous. The value of decreased with an increase in temperature, indicated that the spontaneous nature of the adsorption of AO and CV dyes is inversely proportional to the temperature. The values of other parameters such as enthalpy change and entropy change may be determined from Van’t Hoff equation199:
![]()
The Thermodynamic parameters are listed in Table 6. A positive standard enthalpy change suggests that the interaction of AO and CV by NBATSPED-MWCNT isendothothermic,which supported by the incresing feasibility adsotption AO and CV with the increase in temperature towards NBATSPED-MWCNT.
Comparison of various adsorbent
The performance of the proposed method has been compared with other adsorbents (Table7). The adsorption capacity and contact time for our work are superior to other adsorbents to removal AO. The results indicated that the ultrasound-assisted removal method has are mark able ability to improve the efficiency of dyes removal. The ultrasonic-assisted enhancement of dye removal could be attributed to the high-pressure shock wave sand high speed micro jets during the violent collapse of cavitation bubbles.
Conclusion
It is observed from the present study that the NBATSPED-MWCNT under the sonication is an efficient, fast and sensitive adsorption method for the removal of AO and CV. The influences of experimental parameters on the dyes removal percentages were investigated by experimental design methodology (RSM). The adsorption characteristic was examined with the variations of pH, sonication time, NBATSPED-MWCNTdosage, initial AO and CV concentration. The removal of AO and CV from aqueous solutions in short time (4 min) is feasible with high removal percentage at optimum pH (5.0) which is neutral and is an advantage for the adsorption process. The equilibrium data were best described by the Langmuir model. The process kinetics can be success fully fitted with pseudo-second order model Thermodynamic constants were evaluated using equilibrium constants changing with temperature. The negative values of ΔG°indicate the spontaneity and positive values ofΔH°showed the endothermic nature of AO and CV sorption. The data and methodology presented in this paper might be useful for designing the adsorbent for the treatment of actual effluent. Furthermore, the results of this study encourage the researches and industries to use ultrasound devices for more efficient dye adsorption. The optimized method was successfully applied to real wastewater samples.
Acknowledgement
The author expresses their appreciation to the Science and Research Branch Islamic Azad University, Tehran, Iran for financial support of this work.
References
- Yao L., Zhang L., Wang R., Chou S., Dong Z., J Hazard Mater,2016, 301, 462-470.
- Kim S.Y., Jin M.R., Chung C.H., Yun Y.S., Jahng K.Y., Yu K.Y., , J Biosci Bioeng, 2015,119 , 433-439.
- Mahmoodi N.M., Soltani-Gordefaramarzi S., Sadeghi-Kiakhani M., Environmental monitoring and assessment, 2013,185 , 10235-10248.
- Kaushik P., Malik A., Environmental science and pollution research international,2013, 20 , 2882-2892.
- Wang L., Environmental science and pollution research international, 2013, 20 , 4635-4646.
- Mittal A., J Hazard Mater, 2006,133 , 196-202.
- V.V. Pathak, R. Kothari, A.K. Chopra, D.P. Journal of environmental management, 2015,163 , 270-277.
- Asfaram A., Ghaedi M., Simultaneous determination of cationic dyes in water samples with dispersive liquid-liquid microextraction followed by spectrophotometry: experimental design methodology, New Journal of Chemistry, (2016).
- Asfaram A., Ghaedi M., Goudarzi A., Soylak M., RSC Advances,2015, 5,39084-39096.
- Mall I.D.,. Srivastava V.C,. Agarwal N.K, Journal of Hazardous materials,2007 143 , 386-395.
- Jamshidi M., Ghaedi M., Dashtian K., Hajati S., Bazrafshan A.A., Ultrasonics Sonochemistry,2016 32, 119-131.
- Shukla N.B., Madras G., Journal of Applied Polymer Science,2012, 124 , 3892-3899.
- Dil E.A., Ghaedi M., Ghaedi A., Asfaram A., Jamshidi M., Purkait M.K., Journal of the Taiwan Institute of Chemical Engineers, 2016, 59, 210-220.
- Asfaram A., Ghaedi M., Hajati S., Goudarzi A., RSC Advances,2015, 5, 72300-72320.
- Pourjavadi A.,. Nazari M, Hosseini S.H., RSC Advances,2015, 5 , 32263-32271.
- Li H., An N., Liu G., J. Li, N. Liu, M. Jia, W. Zhang, X. Yuan, J Colloid Interface Sci,2016, 466, 343-351.
- Saratale R.G., Sivapathan S.S., W J.J.,. Kim H.Y, Saratale G.D., Kim D.S., Part A, Toxic/hazardous substances & environmental engineering,2016, 51,164-177.
- Luan J., -X P.. Hou, Liu C., Shi C., X G.-. Li, Cheng H.-M., Journal of Materials Chemistry A,2016, 4 ,1191-1194.
- Agarwal S., Tyagi I., Gupta V.K., Dastkhoon M., Ghaedi M., Yousefi F., Asfaram A., Journal of Molecular Liquids,2016, 219, 332-340.
- Ghaedi M., Ghaedi A.M., dehghanian n., dashtian k., Physical Chemistry Chemical Physics, 2016.
- Mazaheri H., Ghaedi M., Asfaram A., Hajati S., Journal of Molecular Liquids,2016, 219 , 667-676.
- Ghaedi M., Khafri H.Z.,. Asfaram A, Goudarzi A., Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2016, 152 , 233-240.
- Azad F.N., Ghaedi M., Asfaram A., Jamshidi A., Hassani G., Goudarzi A., Azqhandi M.H.A., Ghaedi A., RSC Advances,2016, 6 , 19768-19779.
- Agarwal S., Tyagi I., Gupta V.K., Bagheri A.R., Ghaedi M., Asfaram A.,. Hajati S, Bazrafshan A.A., Journal of Environmental Chemical Engineering,2016, 4 ,1769-1779.
- Asfaram A., Ghaedi M., Yousefi F., Dastkhoon M., Ultrasonics Sonochemistry,2016, 33 (2016) 77-89.
- Roosta M., Ghaedi M., Asfaram A., RSC Advances, 2015, 5, 57021-57029.
- Asfaram A, Ghaedi M., Goudarzi A.,. Rajabi M, Dalton Transactions, 2015, 44, 14707-14723.
- Hajati S., Ghaedi M., Mahmoudi Z., Sahraei R., Part A, Molecular and biomolecular spectroscopy,2015,150 , 1002-1012
- Lee S.L.,. Liew S.W, Ong S.T., Acta chimica Slovenica, 2016,63 , 144-153.
- Tehrani M.S., Zare-Dorabei R., Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2016,160 , 8-18.
- Asfaram A., Ghaedi M., Hajati S., Goudarzi A., Bazrafshan A.A., Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2015, 145, 203-212.
- Dastkhoon M., Ghaedi M., Asfaram A., Goudarzi A., Langroodi S.M., Tyagi I., Agarwal S., Gupta V.K., Response surface optimization, Separation and Purification Technology,2015, 156, Part 2 , 780-788.
- Bazrafshan A.A, Hajati S., Ghaedi M., RSC Advances,2015, 5 , 79119-79128.
- Das P., Banerjee P., Mondal S., Environmental science and pollution research international,2015,22 , 1318-1328.
- Chowdhury S., Chakraborty S., Saha P.D., Environmental science and pollution research international,2013, 20 ,1698-1705.
- Elwakeel K.Z., El-Bindary A.A., El-Sonbati A.Z., Hawas A.R., RSC Advances,2016, 6 , 3350-3361.
- Ansari F., Ghaedi M., Taghdiri M.,. Asfaram A, Experimental design and derivative spectrophotometry method, Ultrasonics Sonochemistry,2016, 33 , 197-209.
- Asfaram A., Ghaedi M.,. Azqhandi M.H.A, Goudarzi A., Dastkhoon M., RSC Advances,2016, 6, 40502-40516.
- Dil E.A., Ghaedi M., Ghaedi A.M., Asfaram A., Goudarzi A., Hajati S., Soylak M., Agarwal S.,. Gupta V.K, Journal of Industrial and Engineering Chemistry,2016, 34 , 186-197.
- Bagheri A.R., Ghaedi M., Hajati S., Ghaedi A.M.,. Goudarzi A, Asfaram A., RSC Advances,2015, 5 ,59335-59343.
- Azad F.N., Ghaedi M., Dashtian K., Hajati S., Pezeshkpour V., Ultrasonics Sonochemistry,2016, 31 (2016) 383-393.
- Jamshidi M., Ghaedi M., Dashtian K., Ghaedi A.M.,. Hajati S, Goudarzi A.,. Alipanahpour E, Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy,2016, 153, 257-267.
- Ghaedi M.,. Khafri H.Z, Asfaram A., Goudarzi A., Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 2016,152 , 233-240.
- Roosta M., Ghaedi M.,. Daneshfar A, Darafarin S., Sahraei R., Purkait M.K., Ultrason Sonochem,2014, 21 ,1441-1450.
- Waldron R., Physical Review,1955, 99 , 1727.
- Nasiri Azad F., Ghaedi M., Dashtian K., Montazerozohori M., Hajati S., Alipanahpour E., RSC Advances,2015, 5 , 61060-61069.
- Naeimi H., Rahmatinejad S., Nazifi Z.S., Journal of the Taiwan Institute of Chemical Engineers, 2016, 58 , 1-7.
- Ioannou Z., Simitzis J., journal of the International Association on Water Pollution Research, 2013,67 ,1688-1694.
- Lim C.K., Bay H.H., Neoh C.H., Aris A., Abdul Majid Z., Ibrahim Z., Environmental science and pollution research international,2013, 20 ,7243-7255.
- Langmuir I., Journal of the American Chemical Society,1918, 40 1361-1403.
- Freundlich U., Die adsorption in lusungen, (1906).
- Temkin M., Pyzhev V., Recent modifications to Langmuir isotherms, (1940).
- Dubinin M., Serpinsky V., Carbon, 19 (1981) 402-403.
- Maghsoudi M., Ghaedi M., Zinali A.,. Ghaedi A.M,. Habibi M.H, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2015, 134 (2015) 1-9.
- El-Bindary A.A., El-Sonbati A.Z., Al-Sarawy A.A., Mohamed K.S., Farid M.A., Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy,2014,136PC ,1842-1849.
- Ho Y.-S, McKay G. , Water research,2000, 34, 735-742.
- Dawood S., Sen T.K., Water research,2012, 46, 1933-1946.
- Ho Y.-S, McKay G., Process biochemistry,1999, 34 , 451-465.
- Weber W.J., Morris J.C., Journal of the Sanitary Engineering Division,1963, 89 , 31-60.
- Yang Y., Wang G., Wang B., Li Z., Jia X., Zhou Q., Zhao Y., Bioresource technology, 2011,102 , 828-834.
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.;Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Monajjemi, M.; Heshmat, M.; Aghaei, H.; Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia, 2007,21 (1)
- Monajjemi, M.;Honarparvar, B. H. ; Haeri, H. ; Heshmat ,M.; Russian Journal of Physical Chemistry C. 2006, 80(1):S40-S44
- Monajjemi, M.; Ketabi, S.; Amiri, A. Russian Journal of Physical Chemistry, 2006, 80 (1), S55-S62
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; K. Zare, K. Journal of Computational and Theoretical Nanoscience. 2013, 10, 10, 2332–2341
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci, 2011, 6, 1496-1500
- Monajjemi, M.; Ghiasi, R.; Seyed Sadjadi, M.A.Applied Organometallic Chemistry,2003,17, 8, 635–640
- Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M.; Sobhanmanesh, A.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2013, 21 47–63
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
- Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. .; Khaleghian M. Journal of Structural Chemistry. 2009, 50, 1, 67-77
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow).2007,72 (6), 652-657
- Ardalan, T.; Ardalan, P.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 687–708
- Mollaamin, F.; Monajjemi, M.; Mehrzad, J. Fullerenes, Nanotubes, and Carbon Nanostructures. 2014, 22: 738–751
- Monajjemi, M.; Najafpour, J.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21(3), 213–232
- Monajjemi, M.; Karachi, N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22: 643–662
- Yahyaei, H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
- Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
- Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296
- Monajjemi, M.; Heshmat, M.; Haeri, HH, Biochemistry (Moscow), 2006, 71 (1), S113-S122
- Monajjemi, M.; Yamola, H.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Mollaamin, F.; Layali, I.; Ilkhani A. R.; Monajjemi, M. African Journal of Microbiology Research .2010, 4(24) 2795-2803
- Mollaamin, F.; Shahani poor, p K. .; Nejadsattari, T. ; Monajjemi, M. African Journal of Microbiology Research. 2010, 4(20) 2098-2108
- Monajjemi, M.; Ahmadianarog, M. Journal of Computational and Theoretical Nanoscience. 2014, 11(6), 1465-1471
- Monajjemi, M.; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 2011, 19(4): 251–261
- Monajjemi, M.; Baheri, H.; Mollaamin, F. Journal of Structural Chemistry.2011 52(1), 54-59
- Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
- Monajjemi, M., Mahdavian, L., Mollaamin, F. Bull. Chem. Soc. Ethiop. 2008, 22(2), 277-286
- Monajjemi, M.; Afsharnezhad, S, Jaafari, M.R..; Mirdamadi, S..; Mollaamin, F..; Monajemi, H. Chemistry .2008, 17 (1), 55-69
- Monajjemi, M.; Mollaamin,F.; Gholami, M. R.; Yoozbashizadeh,H.; Sadrnezhaad, S.K.; Passdar, H.;Main Group Metal Chemistry, 2003, 26, 6, 349-361
- Monajjemi, M.; Azad ,MT.; Haeri, HH.; Zare, K.; Hamedani, Sh.; JOURNAL OF CHEMICAL RESEARCH-S.2003, (8): 454-456
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575–594
- Monajjemi, M.; Noei, M.; Mollaamin, F.Nucleosides, Nucleotides and Nucleic Acids. 2010 29(9):676–683
- Ghiasi, R.; Monajjemi, M.Journal of Sulfur Chemistry .2007, 28, 5, 505-511
- Monajjemi, M.; Ghiasi, R.; Abedi, A. Russian Journal of Inorganic Chemistry.2005, 50(3), 382-388
- Monajjemi, M. .; Naderi, F.; Mollaamin, F.; Khaleghian, M. J. Mex. Chem. Soc. 2012, 56(2), 207-211
- 105. Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013, 10(10), 2473-2477
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. African Journal of Pharmacy and Pharmacology .2010, 4(8), 521 -529
- Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
- Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
- Monajjemi, M.; Honarparvar, B.; Monajemi, H.; Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Ilkhani, Ali R.; Monajjemi, M. Computational and Theoretical Chemistry.2015 1074, 19–25
- Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
- Monajjemi, M., Moniri, E., Panahi, H.A , Journal of Chemical and Engineering Data.2001,1249-1254.
- Mollaamin, F.; Najafpour, J.; Ghadami, S.; Ilkhani, A. R.; Akrami, M. S.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 11 (5), 1290-1298
- Monajjemi, M.; Ghiasi, R.; Ketabi, S.; Passdar, H.; Mollaamin, F.Journal of Chemical Research . 2004, 1, 11.
- Monajjemi, M.; Heshmat, M.; Haeri, H.H. Biochemistry (Moscow).2006, 71, 113-122
- Monajjemi, M.; Heshmat, M.; Aghaei, H.;Ahmadi, R.; Zare, K. Bulletin of the Chemical Society of Ethiopia. 2007, 21, 111–116
- Monajjemi, M., Kharghanian, L., Khaleghian, M., Chegini, H.Fullerenes Nanotubes and Carbon Nanostructures.2014, 22, 8, 0.1080/1536383X.2012.717563
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2011, 49 (5), 561-571
- Amiri, A.; Babaeie, F.; Monajjemi, M.Physics and Chemistry of Liquids. 2008, 46, 4, 379-389
- Monajjemi, M.; Heshmat, M.; Haeri, H.H.; Kaveh, F. Russian Journal of Physical Chemistry A,2006, 80, 7, 1061-1068
- Monajjemi, M.; Moniri, E.; Azizi, Z.; Ahmad Panahi, H. Russian Journal of Inorganic Chemistry. 2005, 50, 1, 40-44
- Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2015, 12, 6, 1030-1039
- Felegari, Z.; Monajjemi, M. Journal of Theoretical and Computational Chemistry. 2015,14, 3, 1550021
- Monajjemi,M; Ghahremani,N; Nikmaram, R;Main group metal chemistry,2002, 25 (12), 733-738
- Monajjemi, M; Azizi, Z; Ghavami, M; Russian journal of inorganic chemistry, 2003, 48 (10), 1551-1559
- Monajjemi, M; Naderi, F; Aghaie, H; RESEARCH JOURNAL OF CHEMISTRY AND ENVIRONMENT, 2007,11 (2), 56-62
- Keivani,M B; Zare, K; Aghaie, M; Aghaie, H; Monajjemi, M; Journal of Chemistry,2010, 7 (1), 105-110
- Mirzaie, S; Monajjemi, M; Hakhamaneshi, MS; Fathi, F; Jamalan, M, EXCLI journal ,2013, 12, 130
- Pournaghdy, M; Giahi, M; Bagherinia, MA; Monajjemi, M; Aghaie, H; Fluid Phase Equilibria,2011, 301 (1), 98-104
- Rajabzadeh, H;Nourouzian, D;Alijanvand, H.H; (…), Saboury, A.A;Moosavi-Movahedi, A.A; Monajjemi, M; Journal of the Iranian Chemical Society,2011, 8 (2), 553-561
- Monajjemi,M; Abedi, A; Passdar, H; Bulletin of the Chemical Society of Ethiopia, 2006,20 (1), 133-142
- Monajjemi, M; Sajadi, MA; Sayadia, R; Kia, M; Ghasemi, G; Main group metal chemistry ,2005, 28 (2), 71-84
- Bakhshi, K; Mollaamin, F; Monajjemi, M; Journal of the Korean Chemical Society,2011, 55 (1), 7-13
- Irani, S; Monajjemi, M; Honarparvar, B; SM Atyabi, SM; Sadeghizadeh, M; International journal of nanomedicine , 2011, 6, 213
- Shabani, M; Monajjemi, M; Aghai, H, Journal of Chemical Research Part S 2003 (5), 249-251
- Amiri, A.; Monajjemi, M.; Ketabi, S.Physics and Chemistry of Liquids2007, 45 (4), 425-433
- Shojaee, S., Monajjemi, M.Journal of Computational and Theoretical Nanoscience. 2015, 12, 3, 449-458
- Esmkhani, R.;Monajjemi, M. Journal of Computational and Theoretical Nanoscience.2015. 12, 4, 652-659
- Monajjemi, M., Seyedhosseini, M., Mousavi, M., Jamali, Z,Journal of Computational and Theoretical Nanoscience. 2015, 23 (3), 239-244
- Ghiasi, R.; Monajjemi, M.; Mokarram, E.E.; Makkipour, P. Journal of Structural Chemistry.2008, 4 , 4, 600-605
- Mahdavian, L.; Monajjemi, M. Microelectronics Journal. 2010,41(2-3), 142-149
- Monajjemi, M.; Baie, M.T.; Mollaamin, F. Russian Chemical Bulletin.2010, 59, 5, 886-889
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Journal of Computational and Theoretical Nanoscience. 2011, 8, 4,763-768
- Darouie, M.; Afshar, S.; Zare, K., Monajjemi, M. journal of Experimental Nanoscience.2013, 8, 4,451-461
- Amiri, A.; Monajjemi, M.; Zare, K.; Ketabi, S.Physics and Chemistry of Liquids. 2006, 44, 4, 449-456.
- Zonouzi, R.;Khajeh, K.; Monajjemi, M.; Ghaemi, N. Journal of Microbiology and Biotechnology. 2013, 23, 1, 7-14
- Ali R. Ilkhani.; Majid Monajjemi, Computational and Theoretical Chemistry.2015, 1074 19–25
- Tahan, A.; Mollaamin, F.; Monajjemi, M. Russian Journal of Physical Chemistry A, 2009, 83 (4), 587-597
- Khalili Hadad, B.; Mollaamin, F.; Monajjemi, M, Russian Chemical Bulletin,2011, 60(2):233-236
- Mollaamin, F.; Monajjemi, M.; Salemi, S.; Baei, M.T. Fullerenes Nanotubes and Carbon Nanostructures, 2011, 19, 3, 182-196
- Mollaamin, F.; Shahani Pour.; K., Shahani Pour, K.; ilkhani, A.R.; Sheckari, Z., Monajjemi, M Russian Chemical Bulletin , 2012 , 61(12), 2193-2198
- Shoaei, S.M.; Aghaei, H.; Monajjemi, M.; Aghaei, M. Phosphorus, Sulfur and Silicon and the Related Elements. 2014, 189, 5; 652-660
- Mehrzad, J., Monajjemi, M., Hashemi, M , Biochemistry (Moscow).2014 , 79 (1), 31-36
- Moghaddam, N.A., Zadeh, M.S., Monajjemi, M. Journal of Computational and Theoretical Nanoscience , 2015 , Vol. 12, No. 3, doi:10.1166/jctn.2015.3736
- Joohari, S.; Monajjemi, M, Songklanakarin Journal of Science and Technology, 2015, 37(3):327
- Rajaian, E., Monajjemi, M., Gholami, M.R, Journal of Chemical Research – Part S, 2002, 6, 1, 279-281
- Ghassemzadeh, L., Monajjemi, M., Zare, K,Journal of Chemical Research – Part S, 2003, 4, 195-199
- Mehdizadeh Barforushi,M.; Safari,S.; Monajjemi,M.;J. Comput. Theor. Nanosci. 2015, 12, 3058-3065.
- Mollaamin,F.; Ilkhani,A.; Sakhaei,N.; Bonsakhteh,B.; Faridchehr,A.; Tohidi,S.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 3148-3154.
- Rahmati,H.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 3473-3481.
- Tarlani Bashiz,R.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 3808-3816.
- Mehrabi Nejad,A.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 3902-3910.
- Monajjemi,M *.; Bagheri,S.; Moosavi,M.S..; Moradiyeh,N.; Zakeri,M.;Attarikhasraghi,N.; Saghayimarouf,N.; Niyatzadeh,G.; Shekarkhand,M.;Mohammad S. Khalilimofrad, Ahmadin,H.; Ahadi,M.; Molecules 2015, 20, 21636–21657;
- Shabanzadeh,E.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 4076-4086.
- Elsagh,A.: Jalilian,H.; Kianpour,E.; Sadat Ghazi Mokri,H.; Rajabzadeh,M.; Moosavi,M.S.; Ghaemi Amiri,F.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 4211-4218.
- Faridchehr,A.; Rustaiyan, A.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 4301-4314.
- Tohidi,S.; Monajjemi,M.; Rustaiyan,A.; J. Comput. Theor. Nanosci. 2015, 12, 4345-4351.
- Ali Akbari Zadeh,M.; Lari,H.; Kharghanian,L.; Balali,E.; Khadivi,R.; Yahyaei,H.; Mollaamin,F.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 4358-4367.
- Dezfooli,S.; Lari,H.; Balali,E.; Khadivi,R.; Farzi,F.; Moradiyeh,N.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 4478-4488.
- Jalilian,H.: Sayadian,M.; Elsagh,A.; Farzi,F.; Moradiyeh,N.; Samiei Soofi,N.; Khosravi,S.; Mohammadian,N.T.; Monajjemi,M.;J. Comput. Theor. Nanosci.2015, 12, 4785-4793.
- Farzi,F.; Bagheri,S.; Rajabzadeh,M.; Sayadian,M.; Jalilian,H.; Moradiyeh,N.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 4862-4872.
- Monajjemi,M.; Nayyer T. Mohammadian J. Comput. Theor. Nanosci. 2015, 12, 4895-4914.
- Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM, 2005, 714 (1), 28, 43-60.
- Joohari*,S.; Monajjemi, M.; Bulgarian Chemical Communications,2015, Volume 47, Number 2 631 – 646.
- Moradiyeh,N.; Zakeri, M.; Attarikhasraghi,N.; Ahadi,M.; Saghayimarouf ,N.; Niyatzadeh , G.; Mahmoodi ,Z.; Shekarkhand,M.; Ahmadin, H.; Monajjemi,M.;J. Comput. Theor. Nanosci. 2015, 12, 5395–5401.
- Zawari, M.; Haghighizadeh, M.; Derakhshandeh,M.; Barmaki ,Z.; Farhami,N.; Monajjemi,M.; J. Comput. Theor. Nanosci.2015, 12, 5472–5478.
- Sadatchoobeh,S.; Monajjemi,M.; J. Comput. Theor. Nanosci. 2015, 12, 5789–5795.
- Shadmani ,N.; MehdizadehBarforushi, M.; Shakibayifar, J.; Elsagh,A.; Zare ,K.; Abbasi,Z.;Khalili1 ,M.S.; Ahmadin ,H.; Rajabzadeh,M.; Sayadian,M.; Monajjemi,M.; J. Comput. Theor. Nanosci.2016, 13, 208–219.
- Shadmani,N.; Monajjemi,M.; Zare,K.; J. Comput. Theor. Nanosci. 2016, 13, 378–387.
- Jamali,Z.; Monajjemi,M.; J. Comput. Theor. Nanosci.2016, 13, 643–651.
- Mirzaei ,R.; Ziglari ,A.; Elsagh,A.; Esmkhani,R.; Monajjemi, M.; J. Comput. Theor. Nanosci.2016, 13, 899–908.
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
- Monajjemi, M.Struct Chem.2012, 23,551–580
- Monajjemi, M.; Chegini, H.; Mollaamin, F.; Farahani, P. Fullerenes, Nanotubes, and Carbon Nanostructures. 2011,19, 469–482
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007,2,1956-1963
- Monajjemi, M.;Baei, M.T.;Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9),1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013,117,1670 −1684
- Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4), 673-692 318
- Nafisi, S.;Monajemi, M.;Ebrahimi, S. Journal of Molecular Structure. 2004,705 (3)35-39
- Fazaeli, R.; Monajjemi, M.; Ataherian, F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Faham, R.; Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures,2012 20, 163–169
- Mollaamin, F.; Najafi, F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures, 201119, 653–667
- I.A. Tan, A.L. Ahmad, B.H. Hameed, J Hazard Mater,2008, 154 , 337-346.
- F.S. dos Anjos, E.F. Vieira, A.R. Cestari, J Colloid Interface Sci, 2002,253 , 243-246.

This work is licensed under a Creative Commons Attribution 4.0 International License.









