Evaluation of Antifungal and Antibacterial Activity and Analysis of Bioactive Phytochemical Compounds of Cinnamomum Zeylanicum (Cinnamon Bark) using Gas Chromatography-Mass Spectrometry
Imad Hadi Hameed1, Huda Jasim Altameme2 and Ghaidaa Jihadi Mohammed3
1College of Nursing, Babylon University, Iraq.
2Department of Biology, Babylon University, Iraq.
3College of Science, Al-Qadisiya University, Iraq.
Corresponding Author E-mail: imad_dna@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320406
Article Received on : May 31, 2016
Article Accepted on : July 19, 2016
Phytochemicals are chemical compounds often referred to as secondary metabolites. Thirty nine bioactive phytochemical compounds were identified in the methanolic extract of Cinnamon bark. The identification of phytochemical compounds is based on the peak area, retention time molecular weight and molecular formula. GC-MS analysis of Cinnamomum zeylanicum revealed the existence of the 6 -Oxa-bicyclo[3.1.0]hexan-3-one, Benzaldehyde, Cyclohexene,4-isopropenyl-1-methoxymethoxymethyl, Benzoic acid- methyl ester, Benzaldehyde dimethyl acetal, Benzenepropanal, Benzylidenemalonaldehyde, 3-Phenylpropanol, Cinnamaldehyde, (E), 2-Propen- 1- ol,3-phenyl, 9-Methoxybicyclo[6.1.0]nona – 2,4,6- triene , 1,3-Bis(cinnamoyloxymethyl)adamantine, Alfa.– Copaene, Naphthalene , 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methyl), Cis – 2-Methoxycinnamic acid, Bicyclo[3.1.1]hept-2-ene,2,6- dimethyl-6-(4-methyl-3-pentenyl), Trans-2-Hydroxycinnamic acid , methyl ester, y-Muurolene, ß-Guaiene, Cadala-1(10),3,8-triene, Isolongifolene,4,5,9,10-dehydro, Cubenol, Tau-Muurolol, Α-Cadinol , Spiro[tricyclo[4.4.0.0(5.9)]decane-10.2oxirane],1-methyl-4-isoprol, 6-Isopropenyl-4,8q-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen, Ethyl9,9-difomylnona-2,4,6,8-tetraenoate, Trans-13-Octadecenoic acid, Tributyl acetylcitrate, 9,12,15-Octadecatrienoic acid ,2,3-dihydroxypropyl ester, 9-Octadecenamide, 17.alfa.-21ß-28,30-Bisnorhopane, 17.alfa.-21ß-28,30-Bisnorhopane, Androstan-3-one,cyclic 1,2-ethanediyl mercaptole , (5α), (4H)4a,5,6,7,8,8a-Hexahydrobenzopyran-5-one-3-carboxamide,2, 4H-Cyclopropa[5´,6´]benz [1´,2´,7,8]azuleno[5,6]oxiren-4-one,8,8a, (22S)-21-Acetoxy-6α,11ß-dihydroxy-16α,17α-propylmethylenediox, (+)-γ-Tocopherol,O-methyl and Stigmasterol. Cinnamomum zeylanicum contain chemical constitutions which may be useful for various herbal formulation as anti-inflammatory, analgesic, antipyretic, cardiac tonic and antiasthamatic. Cinnamomum zeylanicum was highly active against Aspergillus flavus (6.16±0.42). Methanolic extract of bioactive compounds of Cinnamomum zeylanicum was assayed for in vitro antibacterial activity against Pseudomonas aerogenosa, Escherichia coli, Proteus mirabilis, Staphylococcus aureus and Klebsiella pneumonia by using the diffusion method in agar. The zone of inhibition were compared with different standard antibiotics. The diameters of inhibition zones ranged from 6.12±0.52 to 0.39±0.17 mm for all treatments.
KEYWORDS:Antifungal; Antibacterial; Cinnamomum zeylanicum; Gas chromatography-mass spectrometry; Fourier-transform infrared spectroscopy
Download this article as:| Copy the following to cite this article: Hameed I. H, Altameme H. J, Mohammed G. J. Evaluation of Antifungal and Antibacterial Activity and Analysis of Bioactive Phytochemical Compounds of Cinnamomum Zeylanicum (Cinnamon Bark) using Gas Chromatography-Mass Spectrometry. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Hameed I. H, Altameme H. J, Mohammed G. J. Evaluation of Antifungal and Antibacterial Activity and Analysis of Bioactive Phytochemical Compounds of Cinnamomum Zeylanicum (Cinnamon Bark) using Gas Chromatography-Mass Spectrometry. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=19893 |
Introduction
Cinnamomum zeylanicum Blume (Lauraceae), is called true cinnamon. Cinnamon is an evergreen of tropical area reachingabout nine meters high and covered with a smooth, pale bark1-3. It is considered to be the native of Sri Lanka and Malabar Coast of India4,5. Cinnamon mainly contains essential oils and important compounds like cinnamaldehyde, eugenol, cinnamic acid and cinnamate. It has traditionally been used to treat toothache, fight bad breath and treatment common cold6-9. The bark of tree consists of volatile oil, possesses many medicinal properties like antibacterial, anti-oxidant, anti-ulcer, antidiabetic10-12 and antifungal (Bruneton et al., 1998). Cinnamaldehye is the most prevalent with concentration of 6,000 –30,000 ppm13,14. The aims of this study were analysis of chemical compounds of Cinnamomum Zeylanicum (Cinnamon bark) and evaluation of antifungal and antibacterial activity.
Materials and Methods
Collection and preparation of plant material
Cinnamomum zeylanicum (Cinnamon bark) were purchased from local market in Hilla city, middle of Iraq. After thorough cleaning and removal of foreign materials, the Cinnamon bark was stored in airtight container to avoid the effect of humidity and then stored at room temperature until further use15-17.
Preparation of sample
About eighteen grams of methanolic extract of Cinnamomum zeylanicum powdered were soaked in thirty three ml methanol for ten hours in a rotatory shaker. Whatman No.1 filter paper was used to separate the extract of plant18-22. The filtrates were used for further phytochemical analysis. It was again filtered through sodium sulphate in order to remove the traces of moisture.
Gas chromatography – mass spectrum analysis
The GC-MS analysis of the plant extract was made in a (QP 2010 Plus SHIMADZU) instrument under computer control at 70 eV. About 1μL of the methanol extract was injected into the GC-MS using a micro syringe and the scanning was done for 45 minutes. As the compounds were separated, they eluted from the column and entered a detector which was capable of creating an electronic signal whenever a compound was detected23. The greater the concentration in the sample, bigger was the signal obtained which was then processed by a computer. The time from when the injection was made (Initial time) to when elution occurred referred to as the Retention time (RT) 24. While the instrument was run, the computer generated a graph from the signal called Chromatogram. Each of the peaks in the chromatogram represented the signal created when a compound eluted from the Gas chromatography column into the detector. The X-axis showed the RT and the Y-axis measured the intensity of the signal to quantify the component in the sample injected. As individual compounds eluted from the Gas chromatographic column, they entered the electron ionization (mass spectroscopy) detector, where they were bombarded with a stream of electrons causing them to break apart into fragments. The fragments obtained were actually charged ions with a certain mass25-27. The M/Z (mass/charge) ratio obtained was calibrated from the graph obtained, which was called as the Mass spectrum graph which is the fingerprint of a molecule. Before analyzing the extract using Gas Chromatography and Mass Spectroscopy, the temperature of the oven, the flow rate of the gas used and the electron gun were programmed initially. The temperature of the oven was maintained at 100°C. Helium gas was used as a carrier as well as an eluent. The flow rate of helium was set to 1ml per minute. The electron gun of mass detector liberated electrons having energy of about 70eV. The column employed here for the separation of components was Elite 1 (100% dimethyl poly siloxane). The identity of the components in the extracts was assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures. Compounds were identified by comparing their spectra to those of the Wiley and NIST/EPA/NIH mass spectral libraries28.
Determination of antibacterial activity of crude bioactive compounds of Cinnamomum zeylanicum.
The test pathogens (E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia and Staphylococcus aureus) were swabbed in Muller Hinton agar plates. 60μl of plant extract was loaded on the bored wells. The wells were bored in 0.5cm in diameter. The plates were incubated at 37C° for 24 hrs and examined. After the incubation the diameter of inhibition zones around the discs was measured29.
Determination of antifungal activity
Five-millimeter diameter wells were cut from the agar using a sterile cork-borer, and 50 μl of the samples solutions (Cinnamomum zeylanicum) was delivered into the wells. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test microorganisms. Methanol was used as solvent control. Amphotericin B and fluconazole were used as reference antifungal agent30,31. The tests were carried out in triplicate. The antifungal activity was evaluated by measuring the inhibition-zone diameter observed after 48 h of incubation.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) and differences among the means were determined for significance at P < 0.05 using Duncan’s multiple range test (by SPSS software) Version 9.1 .
Results and Discusion
Analysis of phytochemical compounds of methanolic extract of Cinnamomum zeylanicum was carried out by gas chromatography-mass spectroscopy (Table1). The GC-MS chromatogram of the thirty nine peaks of the compounds detected are shown in Figure 1. Chromatogram GC-MS analysis of the methanolic extract of Cinnamomum zeylanicum showed the presence of thirty nine major peaks and the components corresponding to the peaks were determined as follows. The First set up peak were determined to be 6-Oxa-bicyclo[3.1.0]hexan-3-one Figure 2. The second peak indicated to be Benzaldehyde Figure 3. The next peaks considered to be Cyclohexene,4-isopropenyl-1-methoxymethoxymethyl, Benzoic acid , methyl ester, Benzaldehyde dimethyl acetal, -Oxa-bicyclo[3.1.0]hexan-3-one, Benzenepropanal, Benzylidenemalonaldehyde, 3-Phenylpropanol, Cinnamaldehyde, (E), 2-Propen- 1- ol,3-phenyl, 9-Methoxybicyclo[6.1.0]nona – 2,4,6- triene , 1,3-Bis(cinnamoyloxymethyl) adamantine, Alfa.–Copaene, Naphthalene , 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methyl), Cis – 2-Methoxycinnamic acid, Bicyclo[3.1.1]hept-2-ene,2,6- dimethyl-6-(4-methyl-3-pentenyl), Trans-2-Hydroxycinnamic acid , methyl ester, y-Muurolene, ß-Guaiene, Cadala-1(10),3,8-triene, Isolongifolene,4,5,9,10-dehydro, Cubenol, Tau-Muurolol, Α-Cadinol , Spiro[tricyclo[4.4.0.0(5.9)]decane-10.2oxirane],1-methyl-4-isoprol, 6-Isopropenyl-4,8q-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen, Ethyl9,9-difomylnona-2,4,6,8-tetraenoate, Trans-13-Octadecenoic acid, Tributyl acetylcitrate, 9,12,15-Octadecatrienoic acid ,2,3-dihydroxypropyl ester , (z,z,z), 9-Octadecenamide, 17.alfa.-21ß-28,30-Bisnorhopane, 17.alfa.-21ß-28,30-Bisnorhopane, Androstan-3-one,cyclic 1,2-ethanediyl mercaptole , (5α), (4H)4a,5,6,7,8,8a-Hexahydrobenzopyran-5-one-3-carboxamide,2, 4H-Cyclopropa[5´,6´]benz [1´,2´,7,8]azuleno[5,6]oxiren-4-one,8,8a, (22S)-21-Acetoxy-6α,11ß-dihydroxy-16α,17α-propylmethylenediox, (+)-γ-Tocopherol,O-methyl and Stigmasterol Figure 4-40. Five clinical pathogens were selected for antibacterial activity namely, (Klebsiella pneumoniae, Pseudomonas aeroginosa, E.coli, Staphylococcus aeureus and Proteus mirabilis. Maximum zone formation was against klebsiella pneumoniae, Table 2. Methanolic extraction of plant showed notable antifungal activities against Aspergillus niger, Asp. terreus, Asp. flavus, and Asp. fumigatus Table 3. Cinnamomum zeylanicum was very highly active against Aspergillus flavus (6.16±0.42). Aspergillus was found to be sensitive to all test medicinal plants and mostly comparable to the standard reference antifungal drug amphotericin B and fluconazole to some extent.
 |
Table 1: Phytochemical compounds identified in methanolic extract of Cinnamomum zeylanicum. Click here to View table |
Table 2: Zone of inhibition (mm) of test bacterial strains to Cinnamomum zeylanicum bioactive compounds and standard antibiotics.
|
/ Plant Antibiotics |
Bacteria |
||||
|
Proteus mirabilis |
Pseudomonas eurogenosa |
Escherichia coli |
Klebsiella pneumonia |
Staphylococcus aureus |
|
| Plant | 4.92±0.22 | 3.99±0.31 | 5.39±0.22 | 6.12±0.52 | 5.19±0.02 |
| Rifambin |
0.70±0.3 |
0.96±0.11 |
1.00±0.13 |
0.90±0.20 |
0.93±0.50 |
|
Streptomycin |
2.00±0.10 |
1.20±0.18 |
0.97±0.53 |
1.34±0.47 |
1.80±0.38 |
|
Kanamycin |
0.39±0.17 |
0.60±0.33 |
1.00±0.19 |
0.98±0.40 |
0.50±0.12 |
|
Cefotoxime |
0.89±0.6 |
1.40±0.26 |
1.36±0.40 |
0.96±0.39 |
1.90±0.36 |
Table 3: Zone of inhibition (mm) of Aspergillus Spp. test to Cinnamomum zeylanicum bioactive compounds and standard antibiotics.
|
/ Plant Antibiotics |
Aspergillus Spp. |
|||
|
Aspergillus niger |
Aspergillus terreus |
Aspergillus flavus |
Aspergillus fumigatus
|
|
| Plant | 3.00±0.120 | 4.71±0.52 | 6.16±0.42 | 5.19±0.02 |
| Amphotericin B |
2.61±0.270 |
4.28±0.610 |
3.95±0.5 |
4.00±0.820 |
| Fluconazol |
4.79±0.211 |
3.21±0.25 |
2.90±0.451 |
4.70±0.930 |
| Control |
0.00 |
0.00 |
0.00 |
0.00 |
 |
Figure 1: GC-MS chromatogram of methanolic extract of Cinnamomum zeylanicum. Click here to View Figure |
![Figure 2: Structure of 6-Oxa-bicyclo[3.1.0]hexan-3-one present in the methanolic extract of C. zeylanicum using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Eval_Imad_fig2-150x150.jpg) |
Figure 2: Structure of 6-Oxa-bicyclo[3.1.0]hexan-3-one present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
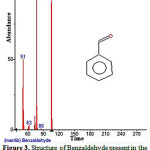 |
Figure 3: Structure of Benzaldehyde present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
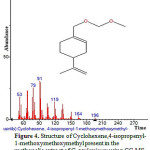 |
Figure 4: Structure of Cyclohexene,4-isopropenyl-1-methoxymethoxymethyl present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
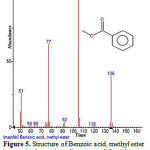 |
Figure 5: Structure of Benzoic acid, methyl ester present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
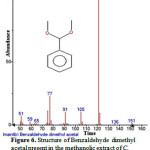 |
Figure 6: Structure of Benzaldehyde dimethyl acetal present in the methanolic extract of C. zeylanicum using GC-MS analysis. |
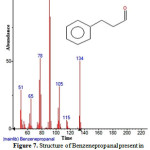 |
Figure 7: Structure of Benzenepropanal present in the methanolic extract of C.zeylanicum using GC-MS analysis |
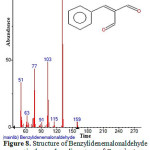 |
Figure 8: Structure of Benzylidenemalonaldehyde present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
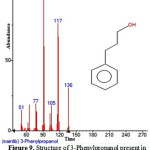 |
Figure 9: Structure of 3-Phenylpropanol present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
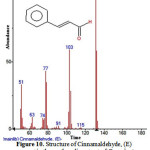 |
Figure 10: Structure of Cinnamaldehyde, (E) present in the methanolic extract of C. zeylanicum using GC-MS analysis. |
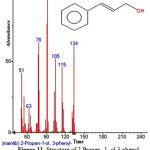 |
Figure 11: Structure of 2-Propen- 1- ol,3-phenyl present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
![Figure 12: Structure of 9-Methoxybicyclo[6.1.0]nona – 2,4,6- triene present in the methanolic extract of C. zeylanicum by using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Eval_Imad_fig12-150x150.jpg) |
Figure 12: Structure of 9-Methoxybicyclo[6.1.0]nona – 2,4,6- triene present in the methanolic extract of C. zeylanicum by using GC-MS analysis. Click here to View Figure |
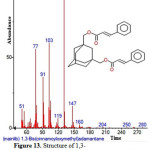 |
Figure 13: Structure of 1,3-Bis(cinnamoyloxymethyl)adamantine present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
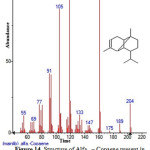 |
Figure 14: Structure of Alfa . – Copaene present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
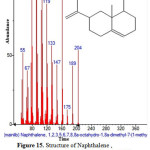 |
Figure 15: Structure of Naphthalene , 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methyl)present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
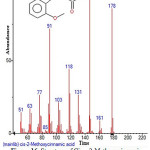 |
Figure 16: Structure of Cis – 2-Methoxycinnamic acid present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
![Figure 17: Structure of Bicyclo[3.1.1]hept-2-ene,2,6- dimethyl-6-(4-methyl-3-pentenyl) present in the methanolic extract of C. zeylanicum using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Eval_Imad_fig17-150x150.jpg) |
Figure 17: Structure of Bicyclo[3.1.1]hept-2-ene,2,6- dimethyl-6-(4-methyl-3-pentenyl) present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
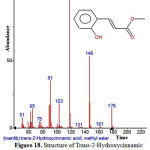 |
Figure 18: Structure of Trans-2-Hydroxycinnamic acid , methyl ester present in the methanolic extract of C. zeylanicum by using GC-MS analysis. Click here to View Figure |
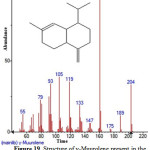 |
Figure 19: Structure of y-Muurolene present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View figure |
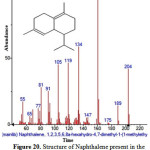 |
Figure 20: Structure of Naphthalene present in the methanolic extract of C. zeylanicum by using GC-MS analysis. Click here to View Figure |
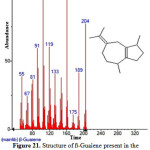 |
Figure 21: Structure of ß-Guaiene present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
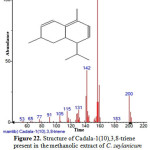 |
Figure 22: Structure of Cadala-1(10),3,8-triene present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
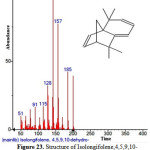 |
Figure 23: Structure of Isolongifolene,4,5,9,10-dehydro present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
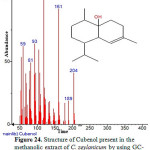 |
Figure 24: Structure of Cubenol present in the methanolic extract of C. zeylanicum by using GC-MS analysis. Click here to View Figure |
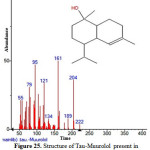 |
Figure 25: Structure of Tau-Muurolol present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
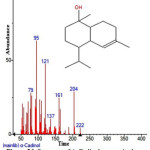 |
Figure 26: Structure of Α-Cadinol present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
![Figure 27: Structure of Spiro[tricyclo[4.4.0.0(5.9)]decane-10.2oxirane] present in the methanolic extract of C. zeylanicum using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Eval_Imad_fig27-150x150.jpg) |
Figure 27: Structure of Spiro[tricyclo[4.4.0.0(5.9)]decane-10.2oxirane] present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
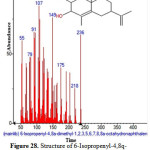 |
Figure 28: Structure of 6-Isopropenyl-4,8q-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
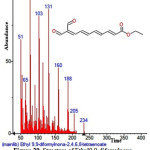 |
Figure 29: Structure of Ethyl9,9-difomylnona-2,4,6,8-tetraenoate present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
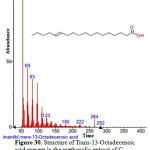 |
Figure 30: Structure of Trans-13-Octadecenoic acid present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
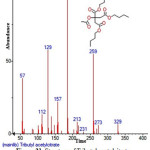 |
Figure 31: Structure of Tributyl acetylcitrate present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
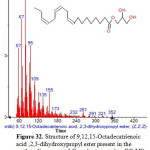 |
Figure 32: Structure of 9,12,15-Octadecatrienoic acid ,2,3-dihydroxypropyl ester present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
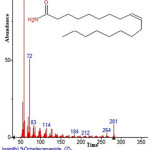 |
Figure 33: Structure of 9-Octadecenamide present in the methanolic seeds extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
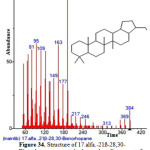 |
Figure 34: Structure of 17.alfa.-21ß-28,30-Bisnorhopane present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
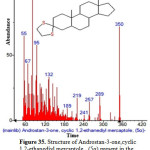 |
Figure 35: Structure of Androstan-3-one,cyclic 1,2-ethanediyl mercaptole , (5α) present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
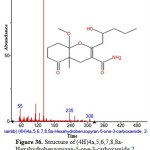 |
Figure 36: Structure of (4H)4a,5,6,7,8,8a-Hexahydrobenzopyran-5-one-3-carboxamide,2 present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
![Figure 37: Structure of 4H-Cyclopropa[5´,6´]benz[1´,2´,7,8]azuleno[5,6]oxiren-4-one,8,8a present in the methanolic extract of C. zeylanicum using GC-MS analysis.](http://www.orientjchem.org/wp-content/uploads/2016/08/Vol32No4_Eval_Imad_fig37-150x150.jpg) |
Figure 37: Structure of 4H-Cyclopropa[5´,6´]benz[1´,2´,7,8]azuleno[5,6]oxiren-4-one,8,8a present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
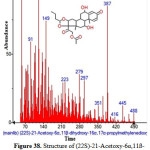 |
Figure 38: Structure of (22S)-21-Acetoxy-6α,11ß-dihydroxy-16α,17α-propylmethylenediox present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
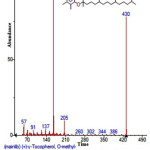 |
Figure 39: Structure of (+)-γ-Tocopherol, present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
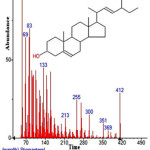 |
Figure 40: Structure of Stigmasterol present in the methanolic extract of C. zeylanicum using GC-MS analysis. Click here to View Figure |
Conclusion
From the results obtained in this study, it could be concluded that Cinnamomum zeylanicum acts possesses remarkable antimicrobial activity, which is mainly due to (E)-cinnamaldehyde. According to these findings, it could be said that the methanolic extract of Cinnamomum zeylanicum acts as antifungal and antibacterial agents.
Acknowlgements
Special thanks to Prof. Abdul-Kareem, Babylon University, Faculty of science for women, for his special care.
References
- Aperis, G.; Myriounis, N.; Spanakis, E.K.; Mylonakis, E. Opin. Investig. Drugs. 2006, 15, 1319-1336.
CrossRef - Brown, R.L.; Mallard, W.G.; Stein, S.E. J. Chromatogr A. 2007, 1157(1-2), 414-421.
CrossRef - Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. In: Pharmacognosy. 43rd ed., Nirali Prakashan, Pune. 1993, 11.46-11.48
- Meena, V.; Sree, S.N.; Surya, P.; Sumanjali, A. RJPBCS. 2012, 3(1), 653-663
- Burt, S. Int. J. Food. Microbiol. 2004; 94, 23-253.
CrossRef - Seyydnejad, S.M.; Niknejad, M.; Darabpoor, I.; Motamedi, H. Am. J. Applied Sci. 2010, 7, 13-16.
CrossRef - Hameed, I.H.; Hamza, L.F.; Kamal, S.A. Journal of Pharmacognosy and Phytotherapy. 2015a, 7(8), 132-163.
CrossRef - Altameme, H.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy. 2015a, 7(10), 238-252.
CrossRef - Al-Jassaci, M.J.; Mohammed, G.J.; Hameed, I.H. International Journal of Pharmaceutical and Clinical Research, 2016, 8(5), 304-315.10.
- Hanafy, M.S.; Hatem, M.E. J. Ethnopharmacol. 1991, 34, 275-278.
CrossRef - Chericoni, S.; Prieto, J.M.; Iacopini, P.; Cioni, P.; Morelli, I. J. Agric Food Chem, 2005, 53(12), 4762-4765.
CrossRef - Mohammed, G.J.; Al-Jassani, M.J.; Hameed, I.H. International Journal of Pharmacognosy and Phytochemical Research, 2016, 8(3), 480-494.
- Diomande, G.D.; Koffi, A.M.; Tonzibo, Z.F.; Bedi, G.; Figueredo, G. Middle East Journal of Scientific Research, 2012, 11(6), 808-813.
- Upadhyay, R.K.; Ahmed, S.; Tripathi, R., Rohtagi, L. J. Med. Plants Res. 2010, 439-445.
- O’Bryan, C.A., Crandall, P.G., Chalova, V.I., Ricke, S.C. J. Food. Sci. 2008, 73, 264-267.
CrossRef - Al-Marzoqi, A.H.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy, 2016, 8(2), 25-48.
CrossRef - Hussein, A.O.; Mohammed, G.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy, 2016, 8(3), 49-59.
CrossRef - Rukayadi, Y., Yong, D., Hwang, J.K. J. Antimicrob Chemother, 2006, 57, 1231-1234.
CrossRef - Altameme, H.J.; Hameed, I.H.; Abu-Serag, N.A. Malaysian Applied Biology, 2015b, 44(4), 47–58.
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Journal of Pharmacognosy and Phytotherapy, 2015b, 7(7), 107-125.
CrossRef - Hussein, H.J.; Hadi, M.Y.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy, 2016, 8(3), 60-89.
CrossRef - Kadhim, M.J.; Mohammed, G.J.; Hameed, I.H. Orient. J. Chem. 2016; 32(2), 10-30.
- Hameed, I.H.; Ibraheam, I.A.; Kadhim, H.J. Journal of Pharmacognosy and Phytotherapy, 2015c, 7(6), 90-106.
CrossRef - Hussein, H.M.; Hameed, I.H.; Ibraheem, O.A. International Journal of Pharmacognosy and Phytochemical Research, 2016, 8(3), 369-385.
- Altameme, H.J.; Hameed, I.H.; Idan, S.A.; Hadi, M.Y. Journal of Pharmacognosy and Phytotherapy. 2015c, 7(9), 222-237.
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Oriental Journal of Chemistry, 2016, 32(2), 20-40.
- Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy, 2016, 8(2), 8-24.
CrossRef - Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Nat. Prod. Res, 2010, 24, 140-151.
CrossRef - Jasim, H.; Hussein, A.O.; Hameed, I.H.; Kareem, M.A. Journal of Pharmacognosy and Phytotherapy, 2015, 7(4), 57-72.
- Hamza, L.F.; Kamal, S.A.; Hameed, I.H. Journal of Pharmacognosy and Phytotherapy, 2015, 7(9), 194-220.
CrossRef - Hameed, I.H.; Altameme, H.J.; Idan, S.A. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2016, 7(2), 1843- 1868.

This work is licensed under a Creative Commons Attribution 4.0 International License.









