Electrodeposition of Platinum Nanoparticles on Polyaniline Modified Electrode and its Electrocatalytic Activity Towards the Oxidation of Methanol
Ali Parsa, Seyed Amir Mirshafieyan, Azadeh Shakeri and Sara Amanzadeh-Salout
Department of Chemistry, College of Science, Yadegar -e- Imam Khomeini (RAH) Shahre-rey Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: aliparsa@iausr.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/320410
Article Received on :
Article Accepted on :
Article Published : 03 Aug 2016
In this research, we reported the electrosynthesis of platinum nanoparticles on Polyaniline (PAni) modified electrode. The modified electrodes were prepared by incorporation of aniline and platinum salt [H2 (PtCl6), 6H2O] in phosphoric acid medium with KCl solution on composite graphite (CG) surface via cyclic voltammetry (CV) method. The synthesized platinum nanoparticles were characterized by using scanning electronic microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). The electro oxidation of methanol was investigated at the surface of these modified electrodes using CV method. It was found that methanol was oxidized with platinum embedded on the surface of PAni modified electrodes during the anodic potential sweep. Moreover, several impact factors such as scan rate, potential and concentration of methanol were studied on the oxidation of methanol toward electrosynthesized electrodes.
KEYWORDS:Electrodeposition; Electrocatalytic oxidation; Platinum nanoparticle; Methanol; Polyaniline
Download this article as:| Copy the following to cite this article: Parsa A, Mirshafieyan S. A, Shakeri A, Salout S. A. Electrodeposition of Platinum Nanoparticles on Polyaniline Modified Electrode and its Electrocatalytic Activity Towards the Oxidation of Methanol. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Parsa A, Mirshafieyan S. A, Shakeri A, Salout S. A. Electrodeposition of Platinum Nanoparticles on Polyaniline Modified Electrode and its Electrocatalytic Activity Towards the Oxidation of Methanol. Orient J Chem 2016;32(4).Available from: http://www.orientjchem.org/?p=19902 |
Introduction
More demand for applying conducting polymers has led to improve the technology of synthesis them as the materials with wide applications. A thin film of conducting polymer on the electrode may improve the electro-catalytic activity and so, it may be increased1-6. The conducting polymers can also cause the easier charge transfer during the electrochemical oxidation of alcohols on Pt surface 7. PAni is one of the most extensively studied conducting polymers due to its easy synthesis, high conductivity, environmental stability and reversible redox catalytic properties 8-15. The metal nanoparticles, such as Pt, on PAni modified electrode show catalytic properties for organic fuel oxidation 16-19. Fuel cells are known as one of the best electrical power to obtain the electrical energy from the combustion of hydrogen or organic compounds, than other sources 20-22. Up to know, Pt is a good catalyst for methanol oxidation, but, because of the poisoning by CO absorption it has low stability. Some research was accomplished to study of CO adsorption and Methanol oxidation on Pt electrodes modified by electrodeposition of PAni film 23-25. In those researches PAni is specified as the context for electro oxidation of hydrogen and methanol by applying the metal catalysts because of increasing the catalytic area for incorporating the metallic particles 26-28. The PAni/Pt nanoparticles electrode has exhibited excellent catalytic activity for methanol oxidation in various mediums 27, 29-32. In this research the Pt nanoparticles were electrodeposited on PAni/CG electrodes with the noble condition of synthesis (during and after the formation of PAni films in phosphoric acid medium and KCl supporting electrolyte), and the electrocatalytic oxidation of methanol on modified electrodes was investigated with the purpose of improving highly active sites of electrodes.
Materials and Methods
Materials
Aniline (Merck, Germany) was distilled under low pressure in the atmosphere and liquid nitrogen at a temperature of 5 ºC until color was maintained. Methanol (MeOH) (BDH Chemicals, UK) was also in pure form. The hexachloro platinic (IV) acid hexahydrate [H2 (PtCl6) 6H2O] (Sigma Chemicals, USA), potassium chloride (KCl) (Fluka Chemicals, Switzerland) and phosphoric acid (H3PO4) (Sigma Chemicals, USA) were used without purification.
Equipment
The electrochemical analysis for the synthesis and characterization of nano-composite electrodes was carried out by a potentiostat/galvanostat compactstat (Ivium Technologies, Netherlands). SEM, model VEGA Corporation (TESCAN) Czechoslovakia equipped with EDX detector has been used to investigate the morphology and size of the Pt nanoparticles on PAni modified electrodes. The composite 2B pencil graphite (CG) (o.d. 1.8 mm) (Staedtler Lumograph, Germany) was used as working electrode against pseudo Ag/AgCl reference electrode.
Procedure
The electrochemical synthesis of PAni was done by 50 mM aniline in 10 mL of 1 M H3PO4 and 0.5 M KCl as a supporting electrolyte, on the working electrode (CG) by sweeping the potential continuously between -0.2 V to +1.00 V vs. Ag/AgCl with a scan rate of 100 mVs-1. Also the Pt particles were incorporated by electrochemical deposition from a solution containing 0.01% wt. [H2 (PtCl6). 6H2O] in 1 M H3PO4 on working electrode.
Results and Discussions
Electrodeposition
Electrodeposition of PAni on CG (PAni/CG)
The cyclic voltammograms (CVs) for the deposition of PAni on CG electrode from the 50 mM aniline in aqueous medium of 10 mL of 1 M H3PO4 and 0.5 M KCl is shown in Fig.1. The three pairs of redox peaks are the result of aniline configuration conversions observed in the potential range of -0.2 V to +1.0 V. The first pair of redox peaks denoted as O3/R3 corresponds to the interconversion of the completely PAni reduced form, leucoemeraldine (LE) state, to the half-oxidized emeraldine base (EB) state. The second pair of redox peaks denoted as O1/R1 is due to oxidation of EB to the completely oxidized pernigraniline (PN) state and vice-versa. The O2/R2 peaks are attributed to oxidation of segments of PAni chain to benzoquinone (Bq) species, whose peak potentials are very close to one another and tend to disappear after several successive scans 33. Fig. 2a shows the surface morphology of PAni film on CG electrode. In the EDX analysis (Fig. 2b) the existence of phosphate ions is shown. This confirms that the doping of aniline with phosphate during polymerization has occurred on the surface of graphite electrode.
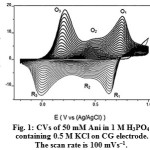 |
Figure 1: CVs of 50 mMAni in 1 M H3PO4 containing 0.5 M KCl on CG electrode. The scan rate is 100 mVs−1. |
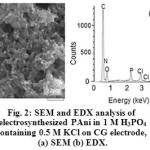 |
Figure 2: SEM and EDX analysis of electrosynthesizedPAni in 1 M H3PO4 containing 0.5 M KCl on CG electrode, (a) SEM (b) EDX. |
Electrodeposition of Pt Nanoparticles on Pani/Cg Electrode (Nppt/Pani/Cg)
The Platinum nanoparticles were incorporated by electrochemical deposition on PAni/CG electrode from H2PtCl6 6H2O solution (0.01% w/w) containing 1 M H3PO4 and 0.5 M KCl (Fig. 3). The CVs reveal one pair of redox peaks that represent the electrodeposition of Pt(IV) to metallic Pt on the surface of PAni/CG film in accordance with reaction (1) 27.
PtCl6 -2 + 4e – → Pt + 6Cl – (1)
Fig. 4a shows a fully homogenized surface of electrode. This confirms that distribution of Pt on PAnifilm is completely uniform. Also it shows the Pt nanoparticles on the PAni/CG electrode (Fig. 4b). EDX spectrum confirms the uniform presence of Pt nanoparticles on the surface of electrode (Fig. 4c).
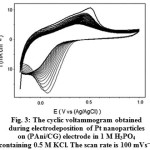 |
Figure 3: The cyclic voltammogram obtained during electrodeposition of Pt nanoparticles on (PAni/CG) electrode in 1 M H3PO4 containing 0.5 M KCl. The scan rate is 100 mVs−1. |
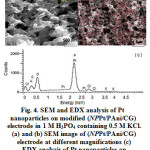 |
Figure 4: SEM and EDX analysis of Pt nanoparticles on modified (NPPt/PAni/CG) electrode in 1 M H3PO4 containing 0.5 M KCl. (a) and (b) SEM image of (NPPt/PAni/CG) electrode at different magnifications (c) EDX analysis of Pt nanoparticles on (PAni/CG) electrode. |
Simultaneous electrodeposition of Pt and PAni on CG (Pt-PAni/CG)
The platinum nanoparticles and PAni were, simultaneously, electrodeposited on CG from a solution of H2PtCl6 6H2O (0.01% w/w), 50 mM aniline, 1 M H3PO4 and 0.5 M KCl. As shown in Fig. 5 the CVs have two redox peaks. In this case, it is seen that PAni is in the conducting emeraldine salt (ES) form which undergoes the oxidation to the PN form, providing electrons for the reduction of PtCl6 -2 to metallic Pt.
Fig. 6a and b shows that, spherical Pt is in context with PAni after the simultaneous deposition. As compared to NPPt/PAni/CG, the distribution and dispersal is less. The absence of Pt in depth of 4 microns was confirmed by EDX analysis (Fig. 6c). As is evident the nanostructure can be changed with monomer displacement, i.e., the conductivity of PAni can play important role in doping and polymerizing of PAni with platinum ions. This result may also be attributed to the higher conductivity and faster charge transfer of PAnifilm in the intermediate oxidation state. It must be pointed out that the catalytic current is greatly in-creased with increase in the amount of deposited Pt34.
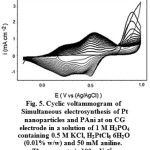 |
Figure 5: Cyclic voltammogram of Simultaneous electrosynthesis of Pt nanoparticles and PAni at on CG electrode in a solution of 1 M H3PO4containing 0.5 M KCl, H2PtCl6 6H2O (0.01% w/w) and 50 mM aniline. The scan rate is 100 mVs−1. |
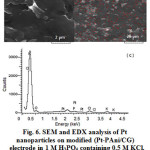 |
Figure 6: SEM and EDX analysis of Pt nanoparticles on modified (Pt-PAni/CG) electrode in 1 M H3PO4 containing 0.5 M KCl. (a) and (b) SEM image of (Pt-PAni/CG) electrode at different magnifications(c) EDX analysis of (Pt-PAni/CG) electrode. |
Electrocatalytic Oxidation of Methanol
Fig. 7a and b shows the CVs of Pt-PAni/CG and NPPt/PAni/CG electrodes in 1 M H3PO4 containing 0.5 M methanol at a scan rate of 100 mVs-1, respectively. The methanol oxidations of Pt-PAni/CG in (Epa = 0.43V) and NPPt/PAni/CG in (Epa= 0.27V) indicate that NPPt/PAni/CG has a relatively superior electrocatalytic performance. The electrochemical oxidation of methanol on Pt surface occurs in two steps 35. . The first step is methanol dehydrogenation in which, methanol is oxidized to become adsorbed COads species. The second step is the removal of COads which proceeded via adsorbed OHads to produce CO2. The adsorbed OHads is produced from water dissociation process on Pt catalyst sites. The overall processes are summarized in reactions (below):
CH3OH → COads + 4H+ + 4e– (2)
H2O → OHads + H+ + e– (3)
OHads + COads → CO2 + H+ + e– (4)
It is known that the integrated intensity of hydrogen absorption represents the number of Pt sites that are available for hydrogen adsorption and desorption 25. To calculate the charge required for hydrogen absorption on the electrode surfaces, we assume that the double-layer charging current is constant over the whole potential range 36. The charge required for hydrogen absorption on the NP PT/PAni/CG surface is 3.14 mC cm-2 which is 1.5 times larger than that required for hydrogen absorption on the Pt-PAni/CG surface (2.07mC cm-2), (Table 1). Comparing the CV results of NP PT/PAni/CG with Pt-PAni/CG electrodes, have indicated a significantly higher oxidation current toward methanol oxidation for the NP PT/PAni/CG electrode. For instance, in Table 1, the maximum anodic peak current densities (Ipa) for NP PT/PAni/CG and Pt-PAni/CG electrodes are 19.32 mA cm-2 and 10.09 mA cm-2, respectively. A high surface area for Pt particles in NP PT/PAni/CG is anticipated due to the uniform distribution of Pt particles into PAni which can twist up to form a spatial 3D dimensional matrix. In addition to this, the -CO2H groups in NP PT/PAni/CG may act as a stabilizer for Pt particles, preventing their aggregation. The PAni/CG matrix acts as a good bed for the deposition of Pt particles, and increases the density of the active sites on the electrode surface.
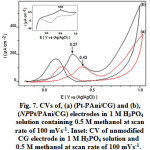 |
Figure 7: CVs of, (a) (Pt-PAni/CG) and (b), (NPPt/PAni/CG) electrodes in 1 M H3PO4 solution containing 0.5 M methanol at scan rate of 100 mVs-1. Inset: CV of unmodified CG electrode in 1 M H3PO4 solution and 0.5 M methanol at scan rate of 100 mVs-1. |
Table 1: CVs data of (NPPt/PAni/CG) and (Pt-PAni/CG) electrode
|
Electrode |
Charge of H2 absorption |
max anodic peak current (Ipa) |
|
|
(NPPt/PAni/CG) (Pt-PAni/CG) |
3.14 mC /cm2 |
19.32 mA /cm2 |
|
|
2.07 mC /cm2 |
10.09 mA /cm2 |
||
Electrocatalytic Oxidation of Methanol
Fig. 7a and b shows the CVs of Pt-PAni/CG and NPPt/PAni/CG electrodes in 1 M H3PO4 containing 0.5 M methanol at a scan rate of 100 mVs-1, respectively. The methanol oxidations of Pt-PAni/CG in (Epa = 0.43V) and NPPt/PAni/CG in (Epa= 0.27V) indicate that NPPt/PAni/CG has a relatively superior electrocatalytic performance. The electrochemical oxidation of methanol on Pt surface occurs in two steps 35. . The first step is methanol dehydrogenation in which, methanol is oxidized to become adsorbed COads species. The second step is the removal of COads which proceeded via adsorbed OHads to produce CO2. The adsorbed OHads is produced from water dissociation process on Pt catalyst sites. The overall processes are summarized in reactions (below):
CH3OH → COads + 4H+ + 4e– (2)
H2O → OHads + H+ + e– (3)
OHads + COads → CO2 + H+ + e– (4)
It is known that the integrated intensity of hydrogen absorption represents the number of Pt sites that are available for hydrogen adsorption and desorption 25. To calculate the charge required for hydrogen absorption on the electrode surfaces, we assume that the double-layer charging current is constant over the whole potential range 36. The charge required for hydrogen absorption on the NPPt/PAni/CG surface is 3.14 mC cm-2 which is 1.5 times larger than that required for hydrogen absorption on the Pt-PAni/CG surface (2.07mC cm-2), (Table 1). Comparing the CV results of NPPt/PAni/CG with Pt-PAni/CG electrodes, have indicated a significantly higher oxidation current toward methanol oxidation for the NPPt/PAni/CG electrode. For instance, in Table 1, the maximum anodic peak current densities (Ipa) for NPPt/PAni/CG and Pt-PAni/CG electrodes are 19.32 mA cm-2 and 10.09 mA cm-2, respectively. A high surface area for Pt particles in NPPt/PAni/CG is anticipated due to the uniform distribution of Pt particles into PAni which can twist up to form a spatial 3D dimensional matrix. In addition to this, the -CO2H groups in NPPt/PAni/CG may act as a stabilizer for Pt particles, preventing their aggregation. The PAni/CG matrix acts as a good bed for the deposition of Pt particles, and increases the density of the active sites on the electrode surface.
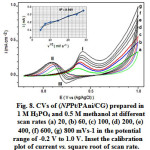 |
Figure 8: CVs of (NPPt/PAni/CG) prepared in 1 M H3PO4 and 0.5 M methanol at different scan rates (a) 20, (b) 60, (c) 100, (d) 200, (e) 400, (f) 600, (g) 800 mVs-1 in the potential range of -0.2 V to 1.0 V. Inset the calibration plot of current vs. square root of scan rate. |
Effect of Methanol Concentration on Electrocatalytic Activity
Figure 9a to d shows the linear sweep voltammograms (LSVs) of NPPt/PAni/CG in 1 M H3PO4 containing different concentration of methanol at the potential range of -0.2 V to1.0 V. A significant enhancement of methanol oxidation has been observed at high concentration of methanol as shown by the increase of the oxidation peak current (Ipa).
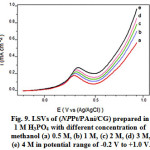 |
Figure 9: LSVs of (NPPt/PAni/CG) prepared in 1 M H3PO4 with different concentration of methanol (a) 0.5 M, (b) 1 M, (c) 2 M, (d) 3 M, (e) 4 M in potential range of -0.2 V to +1.0 V. |
Conclusion
The platinum nanoparticles were successfully electrodeposited onto the surface of PAni/CG electrodes. The NP PT/PAni/CG electrode appeared to have well distribution and dispersal of Pt nanoparticles on the PAni film. The NP PT/PAni/CG electrode exhibited electrocatalytic activity toward methanol oxidation with a higher current density and at lower onset potential. In view of results, the higher concentrations of methanol and higher scan rates of potential have also enhanced the methanol oxidation. According to the extensive studies in this filed, still further research can complement and corroborate these results.
Acknowledgements
This work is supported by the Islamic Azad University,Yadegar -e- Imam Khomeini (RAH) Shahre-rey Branch, Tehran, Iran for Research University (RU) grant -1395/1742:90/07/27
References
- Su, N. Nanoscale Res. Lett. 2015, 10, 1-9 .
CrossRef - Campbell, D. K. Synth. Met. 2001, 125, 117-128 .
CrossRef - Macdiarmid, A. G. Synth. Met. 1987, 21, 79-83 .
CrossRef - Wallace, G. G.; Smyth, M.; Zhao, H. TrAC, Trends Anal. Chem. 1999, 18, 245-251 .
CrossRef - Lange, U.; Roznyatovskaya, N. V.; Mirsky, V. M. Anal. Chim. Acta. 2008, 614, 1-26 .
CrossRef - Morteza, E.; Roya, D. Orient. J. Chem. 2015, 31, 1185-1189 .
CrossRef - Serra, D.; McElwee-White, L. Inorg. Chim. Acta. 2008, 361, 3237-3246 .
CrossRef - MacDiarmid, A.; Chiang, J.; Richter, A.; Epstein, A. Synth. Met. 1987, 18, 285-290 .
CrossRef - MacDiarmid, A.; Yang, L.; Huang, W.; Humphrey, B. Synth. Met. 1987, 18, 393-398 .
CrossRef - Genies, E.; Boyle, A.; Lapkowski, M.; Tsintavis, C. Synth. Met. 1990, 36, 139-182 .
CrossRef - Luzny, W.; Banka, E.; Stochmal-Pomarzanska, E. X-Ray Investigations of Polymer Structures. 2000, 47-50 .
- Ćirić-Marjanović, G. Synth. Met. 2013, 177, 1-47 .
CrossRef - Kajornkavinkul, S.; Punrat, E.; Siangproh, W.; Rodthongkum, N.; Praphairaksit, N.; Chailapakul, O. Talanta. 2016, 148, 655-660 .
CrossRef - Ameen, S.; Shaheerakhtar, M. Orient. J. Chem. 2013, 29, 837-860 .
CrossRef - Udmale, V.; Mishr, D.; Gadhave, R.; Pinjare, D.; Yamgar, R. Orient. J. Chem. 2013, 29, 927-936 .
CrossRef - Han, Y.-K.; Chang, M.-Y.; Ho, K.-S.; Hsieh, T.-H.; Tsai, J.-L.; Huang, P.-C. Sol. Energy Mater. Sol. Cells. 2014, 128, 198-203 .
CrossRef - Farghali, A.; Moussa, M.; Khedr, M. J. Alloys Compd. 2010, 499, 98-103 .
CrossRef - Abaci, S.; Nessark, B.; Boukherroub, R.; Lmimouni, K. Thin Solid Films. 2011, 519, 3596-3602 .
CrossRef - Lemos, H. G.; Santos, S. F.; Venancio, E. C. Synth. Met. 2015, 203, 22-30 .
CrossRef - Andújar, J.; Segura, F. Renew Sust Energ Rev. 2009, 13, 2309-2322 .
CrossRef - Behling, N. H. Fuel Cells. 2013, 423-600 .
- Sharma, R.; Kar, K. K. Electrochim. Acta. 2015, 156, 199-206 .
CrossRef - Wang, Z.; Gao, G.; Zhu, H.; Sun, Z.; Liu, H.; Zhao, X. Int. J. Hydrogen Energy. 2009, 34, 9334-9340 .
CrossRef - Yan, R.; Jin, B. Electrochim. Acta. 2014, 115, 449-453 .
CrossRef - Gyenge, E. PEM Fuel Cell Electrocatalysts and Catalyst Layers. 2008, 165-287 .
- Lei, X.; Guo, X.; Zhang, L.; Wang, Y.; Su, Z. J. Appl. Polym. Sci. 2007, 103, 140-147 .
CrossRef - Sugano, Y.; Yoshikawa, H.; Saito, M.; Tamiya, E. Electrochim. Acta. 2011, 56, 9875-9882 .
CrossRef - Stejskal, J.; Sapurina, I.; Trchová, M. Prog. Polym. Sci. 2010, 35, 1420-1481 .
CrossRef - O’Mullane, A. P.; Dale, S. E.; Day, T. M.; Wilson, N. R.; Macpherson, J. V.; Unwin, P. R. J. Solid State Electrochem. 2006, 10, 792-807 .
CrossRef - Hong, T.-Z.; Xue, Q.; Yang, Z.-Y.; Dong, Y.-P. J. Power Sources. 2016, 303, 109-117 .
CrossRef - Wang, M.; Song, X.; Yang, Q.; Hua, H.; Dai, S.; Hu, C.; Wei, D. J. Power Sources. 2015, 273, 624-630.
CrossRef - Wang, Z.; Shi, G.; Zhang, F.; Xia, J.; Gui, R.; Yang, M.; Bi, S.; Xia, L.; Li, Y.; Xia, L.; Xia, Y. Electrochim. Acta. 2015, 160, 288-295 .
CrossRef - Parsa, A.; Ab Ghani, S. Polymer. 2008, 49, 3702-3708 .
CrossRef - Cai, L.; Chen, H. J. Appl. Electrochem. 1998, 28, 161-166 .
CrossRef - Tripković, A. V.; Popović, K. D.; Lović, J.; Jovanović, V.; Kowal, A. J. Electroanal. Chem. 2004, 572, 119-128 .
CrossRef - Wu, T.-Y.; Li, W.-B.; Kuo, C.-W.; Chou, C.-F.; Liao, J.-W.; Chen, H.-R.; Tseng, C.-G. International Journal of Electrochemical Science. 2013, 10720-10732 .
- Singh, R.; Awasthi, R.; Tiwari, S. Open Catal. J. 2010, 3, 54-61 .
CrossRef - Prabakar, S. J. R.; Kim, Y.; Jeong, J.; Jeong, S.; Lah, M. S.; Pyo, M. Electrochim. Acta. 2016, 188, 472-479 .
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









