Application of Corn Cob as a Natural Adsorbent for the Removal of Mn (Vii) Ions from Aqueous Solutions
*Fariba Norozi and Gholamali Haghdoost
.Department of Chemistry,Kazerun Branch,Islamic Azad University,Kazerun,Iran
*Corresponding Author E-mail: haghdoost1352@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320460
In this Work, We have studied the adsorption of the isotherm of Mn(VII), on Corn cob from aqueous solutions. The effects of pH, initial metal ion concentration, contact time, Corn cobdosage and temperature on the adsorption performance of Corn cob for Mn(VII) ions were examined by batch method. Increasing the Mn(VII) initial concentration declines the Mn(VII) adsorption rate investigated through adsorption Mn(VII) removal at pH=4 condition for tC=60 min in equilibrium-batch mode system. The adsorption equilibrium isotherms were fitted by Freundlich and Langmuir models. It was found that the Langmuir model described the adsorption process better than the Freundlich isotherm,(R2=0.9854), thus indicating the applicability of mono layer coverage of Mn(VII) ion on Corn cob surface.The relationship between thermodynamic parameters was used to predict the absorption process. Thermodynamic analysis showed that the adsorption process was endothermic and spontaneous in nature.
KEYWORDS:Corn cob; Mn(VII) Ions; Adsorption; Isotherm; thermodynamic
Download this article as:| Copy the following to cite this article: Norozi F, Haghdoost G. Application of Corn Cobasa Natural Adsorbent for the Removal of Mn (Vii) Ion from Aqueous Solutions. Orient J Chem 2016;32(4). |
| Copy the following to cite this URL: Norozi F, Haghdoost G. Application of Corn Cobasa Natural Adsorbent for the Removal of Mn (Vii) Ion from Aqueous Solutions. Orient J Chem 2016;32(4). Available from: http://www.orientjchem.org/?p=20621 |
Introduction
Manganese is the second most abundant metal in nature. Its most common mineral is pyrolusite (MnO2). It is an essential metal for the human system and enzymes activation. It has a variety of applications in ceramics, dry battery cells and electrical coils; it is also an alloying element1-2. In addition to the disposal of untreated discharge from above the applications into water, another major source of pollution of the manganese is burning of coal and oil. Because of the toxicity and non-degradable nature of metallic species, scientists the world over have carried out significant work on their removal from aqueous solutions and industrial effluents. Membrane filtration, chemical precipitation, ion exchange, silica, adsorption on activated carbon 3-5, etc., are among the commonly used methodologies for this purpose. Corn cob, flamboyant pods 6-7, apricot stone8-10, almond shell, nut shell, peach stone 11, oat hulls,coconut husk 12, coconut shell 13-14, hazelnut shell 15,grape seed 16, olive stone and Rosa canina sp. Seeds17 have been used for activated carbon production.In the present study, Corn cob used as an adsorbent to remove Mn(VII) from aqueous solutions.The effects of different parameters including pH, initial metal ion concentration and temperature were investigated. Langmuir and Freundlich isotherms were used to analyze the equilibrium data.
Materials and Methods
Corn cob used in this study was collected from Iran. All the Chemicals used were of analytical grade and obtained from Aldrich chemical, USA. The aqueous solution of Mn(VII) was prepared by dissolving KMnO4 in double distilled water. The pH of the solution was adjusted using 0.01 N HCl or 0.01 N KOH.
Batch Adsorption Experiments
The effects of experimental parameters such as initial metal ion concentration (50 mg/L), pH (2–8) and temperature (25–650C) on the adsorptive removal of Mn(VII) ions were studied in a batch mode of operation for a specific period of contact time for 20-70 min. All adsorption experiments were conducted in 250 mL conical flasks, adding 100 mL of Mn(VII) solution (with desired concentration and pH) and 0.25-2g of Corn cob, and then mechanically agitating them in an isothermal water bath shaker at 220 rpm for the wished temperature.The filtrate was analyzed by an UV spectrophotometer (Perkin Elmer, Lambda 25)for Mn(VII) content.
The amount of adsorbed Mn(VII) ions per gram Corn cobatequilibrium, qe (mg/g), and the removal percentage, (% A),were calculated using the following equations:

Where C0 and Ce are the initial and equilibrium concentrations of Mn(VII) respectively (mg/L). V is the volume of Mn(VII) solution (L) and m is the weight of Corn cob used (g) 13-18.
Results and Discussion
Effect of pH
Solution pH is one of the most important parameters to determine the adsorption property of an adsorbent that it controlled the kind and amount surface charge of the adsorbent 19. Also amount pH reveals degree of ionization and speciation of adsorbente. Table1 illustrate the effect of the pHof the solution on the adsorption percentage of Mn(VII) ions adsorbed onto Corn cob. It is evident that increasing solution pH significantly increases the percent removal in the pH range from 2 to 8 concentration of 50 mg L-1.
As a result, Manganese hydroxylspecies may participate in the adsorption and/or precipitation onto the adsorbent structure. The maximum removal percentage occurs at pH =4 and hence it was taken as the optimal value for further adsorption studies.
Effect of Corn Cob Dosage
The effect of Corn cob dosage on the percentage Mn(VII) removal at different initial Mn(VII) ions concentrations shown in Table1. The experimental results revealed that Mn(VII) ions percent increases up to the optimum dosage, then percent removal dosage not change with Corn cob dosage. As expected, the equilibrium concentration decreases with increasing adsorbent doses for a given initial Mn(VII) concentration, because for a fixed initial solute concentration, increasing adsorbent dose provides a greater surface area or more adsorption sites. After optimum dosage, all active sites are entirely exposed and the adsorbent surface is saturated.
Effect of Temperature
Temperature has a pronounced effect on the removal of pollutant species from aqueous solutions with most adsorption processes being exothermic in nature 15-20. Investigation for the present process also revealed its exothermic nature. The removal of Mn(VII) decreased from 74.08 % to 52.71% by increasing the temperature from 25 to 650C (Table1). The variation in the removal may be a result of the enhanced escaping tendency of pollutant species at increasing temperatures. The possibility of increased solubility at higher temperatures and hence a lower adsorption can also not be ruled out.
Effect of Contact Time
The effect of contact time (tc), on the adsorption percentage of Mn(VII) ion onto Corn cob was studied and shown in Table1. A rather fast uptake occurs during the first 50 min of the adsorption process followed by a slower stage as the adsorbed amount of Mn(VII) reaches its equilibrium value. The removal curves are single, smooth, and continuous, indicating the possibility of the formation of monolayer coverage of Mn(VII) at the outer surface of Corn cob. According to the results, the equilibrium time was fixed at 65 min. for the rest of the batch experiments to make sure that equilibrium is reached.
Table 1: The effect of pH, Corn cobDosage, temperature and Contact Time on Mn(VII)adsorbed by Corn cob
| pH | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Mn(VII)Removal% | 80.4 | 81.9 | 85.23 | 78.3 | 71 | 64.08 | 60.2 |
| Corn cobDosage(g) | 0.25 | 0.5 | 0.75 | 1 | 1.25 | 1.5 | 2 |
| Mn(VII)Removal% | 67.63 | 69.39 | 76.2 | 88.06 | 88.06 | 88.06 | 88.06 |
| Temperature(K) | 298 | 308 | 318 | 328 | 338 | – | – |
| Mn(VII)Removal% | 74.08 | 67.21 | 64.89 | 59.05 | 52.71 | – | – |
| Time(min) | 20 | 30 | 40 | 50 | 60 | 70 | – |
| Mn(VII)Removal% | 43 | 51 | 58.3 | 68.3 | 68.41 | 68.41 | – |
| C0(Mn+7) (mg/L) | 20 | 30 | 40 | 50 | 60 | – | – |
| Mn(VII)Removal% | 55 | 64 | 72.5 | 76.6 | 79.8 | – | – |
Adsorption Isotherm
An adsorption isotherm is characterized by certain constant values, which express the surface properties of the adsorbent, and could also be used to compare the adsorptive capacities of the adsorbent for different pollutants. Equilibrium data can be analyzed using the Langmuir and Freundlich isotherm models. The Langmuir isotherm assumes monolayer on a homogeneous surface without any interaction between adsorbed ions and with uniform binding sites and equivalent sorption energies. The linear form of Langmuir equation is expressed as 21:

Where qm (mgg-1) is the maximum adsorption capacity corresponding to complete monolayer coverage and KL (L/mg) is the Langmuir constant related to adsorption capacity and energy of adsorption.The slope and intercept of plot of 1/qe versus 1/Ceat different temperatures, were used to calculated L and qm (Fig1A). Langmuir isotherm parameters fit (Table 2) for Mn(VII) adsorption on Corn cob yielded isotherm that were in good agreement with observed behavior.
The Freundlich equation is an empirical equation based on adsorption on a heterogeneous surface. This isotherm is applicable to both monolayer (chemisorption) and multilayer adsorption with interaction between adsorbed molecules. The linear form of the Freundlich isotherm model is described as;

KF (L/g) and n are the Freundlich constants related to adsorption capacity and adsorption intensity 22.
In the present study a concentration of Mn+7 over the range 20 to 60 mg L-1 was examined and the Ce and qe Table 1).
The values of KF and n are determined from the intercept and slope of a plot of Lnqe versus LnCe at temperature from 298K, were used to calculated the KF and n (Fig1B), and are listed in Table 2.
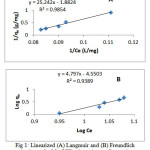 |
Figure 1: Linearized (A) Langmuir and (B) Freundlich isotherm for Mn(VII) adsorption on Corn cobparticles at different temperatures. Click here to View Figure |
Table 2: Langmuire and Freundlich isotherm model parameters and correlation coefficients for adsorption of Mn(VII) ion on Corn cobparticles Isotherm.
| Isotherm models | Mn (VII)conestant | isotherm modelsconestant | Mn (VII) |
| Langmuire: | Freundlich: | ||
| qm(mg/g) | 0.53 | KF(mg/g)(L/mg)1/n | 0.0000282 |
| KL(L/mg) | 0.075 | n | 0.21 |
| R2 | 0.9854 | R2 | 0.9389 |
The essential characteristic separation constant factor, RL, for the Langmuire adsorption is defined as follows:

The value of RL illustrate the shape of the isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0<RL<1) or irreversible (RL=0). The RL values between 0 and 1 indicate favorable adsorption21.
In the present study, the calculated values of RL is observed to be in the 0.4 – 0.18.
Thermodynamic Parameters
The thermodynamic parameters of adsorption process can be determined from the variation of thermodynamic equilibrium constant, Ko. Which Ko is defined as follow 23.

Where and are the activity of adsorbed Pb2+ and the activity of Pb2+ in solution at equilibrium.
The adsorption standard free energy change (ΔG0), average standard enthalpy change (ΔHo), and average standard entropy change (ΔSo) were calculated using the equations (7)and (8)24-25

The values of and are determined from the intercept and slope of a plot of LnK0 versus (figure 2). The thermodynamic parameters are listed in Table 3. Negative values of ΔG0 indicate spontaneous adsorption and the degree of spontaneity of the reaction increases with increasing temperature. The positive standard enthalpy change suggests that the adsorption Mn(VII) by Corn cob is endothermic, which is supported by the increasing adsorption of Mn(VII) with the increase in temperature. The positive standard entropy change indicated increased randomness at the adsorbent-solution interface during the adsorption of Mn(VII) onto Corn cob.
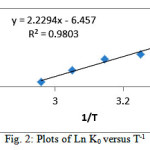 |
Figure 2: Plots of LnK0 versus T-1 |
Table 3: Thermodynamic parameters for adsorption of Mn(VII) onto Corn cob
| Temperature (K) | ΔG0 (kJ mol-1) | ΔH0 (J mol-1)1) | ΔSo (J mol-1K– |
| 298 | -2.6 | 22.631 | 79 |
| 308 | -1.85 | 22.631 | 79 |
| 318 | -1.62 | 22.631 | 79 |
| 328 | -1.00 | 22.631 | 79 |
| 338 | -0.31 | 22.631 | 79 |
Conclusion
In this work, Corn cob was used as an adsorbent to remove Mn(VII) from aqueous solutions. Increasing the Mn(VII) initial concentration declined the Mn(VII) adsorption rate, investigated through adsorption Mn(VII) removal at pH=4 condition for tC=60 min in equilibrium-batch mode system. The removal curves are single, smooth, and continuous, indicating the possibility of the formation of mono layer coverage of Mn(VII) at the outer surface of Corn cob. The variation in the removal may be the result of the enhanced escaping tendency of pollutant species at increasing temperatures. The possibility of increased solubility at higher temperatures and hence a lower adsorption can also not be ruled out. Maximum adsorption at acidic pH indicates that low pH leads to an increase in H+ ions on the Corn cob surface,resulting in significantly strong electrostatic attraction between positively charged Corn cob surface and manganese ions.
As a result, Manganese hydroxyl species may participate in the adsorption and/or precipitation onto the adsorbent structure. The maximum removal percentage occurs at pH =4 and hence, it was taken as the optimal value for further adsorption studies.
Increasing the initial Mn(VII) concentration decreased the Mn(VII) adsorption rate but the adsorption capacity increased. The Langmuir isotherm gave a better fit than the Freundlich isotherm, as indicated by the high values of the correlation coefficients (R2 =0.9854), thus indicating the applicability of mono layer coverage of Mn(VII)ion on Corn cob surface. The calculated RL values versus initial Mn(VII) concentration (0.4- 0.18), indicate that the Langmuire adsorption of Mn(VII) onto Corn cobis favorable.
Thermodynamic analysis showed that the adsorption process was endothermic and spontaneous in nature. Negative values of ΔG0 indicate spontaneous adsorption and the degree of spontaneity of the reaction increases with increasing temperature. The positive standard enthalpy change suggests that the adsorption Mn(VII) by Corn cob is endothermic, which is supported by the increasing adsorption of Mn(VII) with the increase in temperature. The positive standard entropy change indicated increased randomness at the adsorbent-solution interface during the adsorption of Mn(VII) onto Corn cob.
Accknowledgment
The authors express their appreciation to the Graduate School and Research Council of the Islamic Azad University, Kazerun for the financial support of this work.
References
- Bessbousse, H., Rhlalou, T., Lebrun, L.J. Membr. Sci. 2008,307, 249-259.
CrossRef - Gonza´lez-Mun˜ oz, M.J., Rodrı´guez, M.A., Luque, S., lvarez, JR.Desalination.2006, 200, 742-744.
- Kiefer, R., Kalinitchev, A.I., Ho¨ ll, WH. React. Funct. Polym. 2007, 67, 1421-1432.
CrossRef - Passos, C.G., Lima, E.C., Arenas, L.T., Simon, N.M., daCunha, B.M., Brasil, J.L., Costa, T.M.H.Benvenutti, EV. ColloidsSurf.2008,316, 297-306.
CrossRef - Amuda, O.S., Giwa, A.A., Bello, I.A.Biochem. Eng. J. 2007,36, 174-181.
CrossRef - Sun, Y.,Webley, P.A.Chem. Eng.2010,162, 883-892.
CrossRef - Tsai, W.T., Chang, C.Y., Wang, S.Y., Chang, C.F., Chien, S.F., Sun, H.F. Technol.2001,78, 203-208.
- Vargas, A.M.M., Garcia, C.A., Reis, E.M., Lenzi, E., Costa, W.F. Almeida, V.C.Chem. Eng. J. 2010,162,43-50.
CrossRef - Gergova, K.,Eser, S.Carbon. 1996,34,879-888.
CrossRef - Savova, D., Apak, E., Ekinci, E., Yardım, F., Petrov, N., Budinova, T., Razvigorova, M.,Minkova, V. Biomass Bioenergy.2001,21,133-142.
CrossRef - Heschel, W.,Klose, E.Fuel. 1995,74,1786-1791.
CrossRef - Tan, I.A.W., Ahmad, A.L. Hameed, B.H.Chem. Eng. J. 2008,137,462-470.
CrossRef - Mozammel, H.M., Masahiro, O.,Bahattacharya, S.C.Biomass Bioenergy. 2002,22,397-400.
CrossRef - Hu, Z., Srinivasan, M.P. Yaming, N. Carbon. 2001,39, 877-886.
CrossRef - Gergova, K., Petrov, N.,Eser, S. Carbon. 1994,32, 693-702.
CrossRef - Sabio, M.M.Reinoso, F.R.Colloids Surf. 2004,241, 15-25.
- Gurses, A., Dogar, C., Karaca, S., Ackyldz, M.,Bayrak, R.Journal of Hazardous Materials.2006,131, 254-259.
CrossRef - Benguella, B.,Bbenaissa, H. Water Research. 2002,36, 2463-2470.
CrossRef - Jimenez-Reyes, M.,Solache-Rios, M.Journal of Hazardous Materials. 2010,180, 297-303.
CrossRef - Netpradit, S., Thiravetyan, P.,Towprayoon, S. Water Res. 2003, 37, 763-772.
CrossRef - Langmuir, I. Journal of the American Chemical Society. 1916,148, 2221-2226.
CrossRef - Celebi, O., Uzum, C., Shahwan, T.,Erten, H.N. Journal of Hazardous Materials. 2007,148, 761-767.
CrossRef - Calvet, R.Environmental Health Perspectives. 1989,83, 145-177.
CrossRef - Hashemian, S.; Parsaei, Y. Orient. J. Chem. 2015, 31(1), 177-184.
CrossRef
- Mohammadkhani, S.; Gholami, M.R.; M. Aghaie. Orient. J. Chem. 2016, 32(1), 591-599.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









