Synthesis of α-Diazoketones by The Action of Diazo-N-Octane on 3-Methoxy Cinnamoyl Chloride
Devendra Kumar Gangwar* and A. K. Agarwal
Department of Chemistry, Bareilly College, Bareilly - 253 005 (India)
Corresponding Author E-mail : gangwardevendra2@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320345
Article Received on : March 07, 2016
Article Accepted on : April 17, 2016
The reaction of 3-methoxy cinnamoyl chloride (1 mol) with diazo-n-octane (2 mol and 3 mol) gives 1-diazo-1-n-heptyl-4-(3-methoxy) phenyl but 3-ene 2-one and 4-(3-methoxy) phenyl-3-n-heptyl 1-diazoacetyl-5-n-heptyl pyrazoline. The diazoketones were characterised by various physico-chemical techniques.
KEYWORDS:Diazo-n-octane; 3-methoxy cinnamoyl chloride
Download this article as:| Copy the following to cite this article: Gangwar D. K, Agarwal A. K. Synthesis of α-Diazoketones by The Action of Diazo-N-Octane on 3-Methoxy Cinnamoyl Chloride. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Gangwar D. K, Agarwal A. K. Synthesis of α-Diazoketones by The Action of Diazo-N-Octane on 3-Methoxy Cinnamoyl Chloride. Orient J Chem 2016;32(3). Available from: http://www.orientjchem.org/?p=16992 |
Introduction
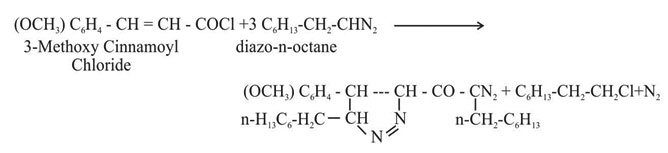
A number of methods for synthesis of a-diazo ketones by the reaction of higher diazoalkanes with carboxylic acid chlorides or acid anhydrides1-4. The field of synthesis of new a-diazoketones is too wide5-7. The study of literature shows that a very little work has been done with higher diazo alkanes8-10. By using different amount of diazoalkane, it is possible to attack one or both the sites present in it. By doing so, it is possible to compare the reactivity of these sites11. In most cases the acid chloride12 group is attacked first and the other side afterwards. Thus starting from 3-methoxy cinnamoyl chloride (1 mol) and diazo-n-octane (2 mol and 3 mol) 1-diazo-1-n- heptyl-4-(3-methoxy) phenyl 3-ene-2-one and 4-(3-methoxy) phenyl-3-n-heptyl 1-diazoacetyl-5-n-heptyl pyrazoline were synthesized following the method of Arndt-Eistert.
Above diazoketones were light yellow viscous liquids. The easily removal diazo group present in them, prevented their purification by distillation even under vaccum.
Experimental
Synthesis of 1-diazo-1-n- heptyl-4-(3-methoxy) phenyl 3-ene-2-one
It was prepared by using 3-methoxy cinnamoyl chloride (2.8 g, 1 mol) on pre-estimated diazo-n-octane (4.32 g, 2 mol) at 00C. The reaction mixture was then kept at 00C overnight. On removal of ether at low temperature the diazoketone was obtained as yellow mobile liquid which contained nitrogen. 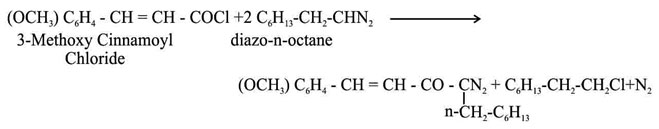
Synthesis of 4-(3-methoxy) phenyl-3-n-heptyl 1-diazoacetyl-5-n-heptyl pyrazoline
The diazoketone so obtained was characterised by elemental analyses and its reactions with 2,4-dinitrophenyl hydrazine, benzoic acid, phenol and dry hydrochloric acid. It was prepared by using 3-methoxy cinnamoyl chloride (2.8 g, 1 mol) on pre-estimated diazo-n-octane (6.48 g, 3 mol) at 00C. The reaction mixture was then kept at 00C overnight. On removal of ether at low temperature the diazoketone was obtained as yellow mobile liquid which contained nitrogen.
Results and Discussion
The diazoketone so obtained was characterised by elemental analyses and its reactions with 2,4-dinitrophenyl hydrazine, benzoic acid, phenol and dry hydrochloric acid. The elemental analyses and IR spectral studies were carried out at CDRI Lucknow.
Characterisation of 1-diazo-1-n- heptyl-4-(3-methoxy) phenyl 3-ene-2-one
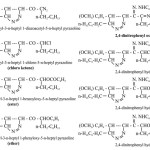
The diazoketone with an aqueous alcoholic sulphuric acid solution of 2, 4- dinitrophenyl hydrazine gave a 2,4-dinitrophenyl osazone as an orange solid, which after crystallisation from ethanol, melted at 1580C (Found C= 55.78%, H = 4.55%, N=17.32%, C30H32O9N8, required C= 55.55%, H = 4.93%, N=17.28%) absorbed frequencies IR(KBr) : 3435 (-NH), 1632(C=N), 1620 (-C6H5), 1355(C-NO2), 970(-CH=CH-), 724 Cm-1 (CH2 rock in-C7H15). With dry HCl formed chloroketone afforded 2, 4- dinitrophenyl hydrazone. With benzoic acid gave an ester, afforded 2,4 nitro phenyl hydrazone. (Found C= 63.12%, H = 5.32%, N=9.25%, C31H34O7N4, required C= 64.80%, H = 5.92%, N=9.75%) absorbed frequencies IR(KBr) : 3350
(-NH), 1720 (C=O), 1615 (C=N), 1580 (-C6H5), 1320(C-NO2), 965(-CH=CH-), 724 Cm-1 (CH2 rock in-C7H15). With phenol gave an ether, afforded 2,4 nitro phenyl hydrazone.
 |
Scheme 1 Click here to View Scheme |
Characterisation of 4-(3-methoxy) phenyl-3-n-heptyl 1-diazoacetyl-5-n-heptyl pyrazoline
The diazoketone with an aqueous alcoholic sulphuric acid solution of 2, 4- dinitrophenyl hydrazine gave a 2,4-dinitrophenyl osazone as an orange solid, which after crystallisation from ethanol melted at 1320C (Found C= 57.76%, H = 6.20%, N=17.33%, C38H48O9N10, required C= 57.86%, H = 6.09%, N=17.56%) absorbed frequencies IR(KBr) : 3440 (-NH), 1632(C=N), 1620 (-C6H5), 1332(C-NO2), 725 Cm-1 (CH2 rock in-C7H15). With dry HCl formed chloroketone afforded 2, 4- dinitrophenyl hydrazone. With benzoic acid gave an ester, afforded 2,4 nitro phenyl hydrazone. (Found C= 65.14%, H = 7.25%, N=11.12%, C39H50O7N6, required C= 65.54%, H = 7.00%, N=11.76%) absorbed frequencies IR(KBr) : 3350 (-NH), 1720 (C=O), 1625 (C=N), 1590 (-C6H5), 1320(C-NO2), 724 Cm-1 (CH2 rock in-C7H15). With phenol gave an ether, afforded 2,4 nitro phenyl hydrazone.
 |
Scheme 2 Click here to View Scheme |
Acknowledgement
The authors are thankful to the principal, Bareilly College, Bareilly and Head, Department of Chemistry for providing necessary facilities and also CD.R.I, Lucknow for various infra red spectra and nitrogen estimation.
References
- Sudrik, S.G.; Sharma J.; Chavan. V.B.; Chaki, N.K. Sonwane, H.R.; Vijaymohanan K.P. Org. Lett. 2006, 8, 1089.
CrossRef - Toma, T., Shimokawa, J., Fukuyama, T., Org. Lett., 2007, 9, 3195-3197.
CrossRef - Yadav J.S., Reddy, B.V.S., Rao Y.G. Narsalah A.V. Tetrahedron Lett.2008 49, 2381.
CrossRef - Taber D.F.; Tian, W.; J. Org. Chem; 2007, 72, 3207-3210.
CrossRef - Ceasar J., Dolenc, S., Tetrahedron Lett., 2001, 42, 7099-7102.
CrossRef - Pace. V.; Verniest, G.; Sinisterra, V; Akantara, A.R.; Kimbe, N.D., J. Org. Chem 2010, 75, 5760-5763.
CrossRef - Chakraborti S.; Agarwal, A.K.; Orient J. Chem. 2010, 26, 1573-1575.
- Arndt F., Eistert B., Partale W., Ber. 1927, 60, 1364.
- Gupta S.; Agarwal, A.K.; Garg, P.; Asian J. Chem, 2010, 22, 2939-2942.
- Saxena. N.; Agarwal, A.K.; Chandra, A.; Imam, S.G.; Orient J. Chem. 2013, 29, 1193-1195.
CrossRef - Gupta, S., Garg, P., Agarwal, A.K., Orient J. Chem., 2009, 25, 457-458.
- Saxena N.; Chandra A.; Imam, S.G.; Chandra, G.; Orient J. Chem., 2013, 29, 1085-1088.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









