Exfoliation Dynamics of Laponite Clay in Aqueous Suspensions Studied by NMR Relaxometry
Anastasia Karpovich, Maria Vlasova, Natalya Sapronova, Valentin Sukharev and Victor Ivanov*
Moscow Institute of Physics and Technology (State University), Institutskii per. 9, Dolgoprudny, Moscow Region, Russia, 141700
Corresponding Author E-mail: ivanov.vv@mipt.ru
DOI : http://dx.doi.org/10.13005/ojc/320346
Article Received on : May 06, 2016
Article Accepted on : June 16, 2016
Article Published : 08 Jun 2016
The interaction between Laponite and other constituents in complex systems greatly depends on its available surface area. We report a study of exfoliation dynamics of Laponite in aqueous suspensions by NMR relaxometry. It showed that Laponite particles exfoliate to the same extent in a concentration range of 0.5-3% w/w. Faster increase of specific wetted surface area of Laponite particles in more concentrated suspensions suggests faster exfoliation of disc-shaped Laponite platelets from the initial layered structure.
KEYWORDS:NMR relaxometry; Laponite; exfoliation; clays
Download this article as:| Copy the following to cite this article: Karpovich A, Vlasova M, Sapronova N, Sukharev V, Ivanov V. Exfoliation Dynamics of Laponite Clay in Aqueous Suspensions Studied by NMR Relaxometry. Orient J Chem 2016;32(3). |
| Copy the following to cite this URL: Karpovich A, Vlasova M, Sapronova N, Sukharev V, Ivanov V. Exfoliation Dynamics of Laponite Clay in Aqueous Suspensions Studied by NMR Relaxometry. Orient J Chem 2016;32(3).Available from: http://www.orientjchem.org/?p=17036 |
Introduction
Synthetic hectorite Laponite is one of the most used materials for studies of exfoliating clays due to its high dispersiblity in water, narrow size distribution and uniform shape of its individual disc-shaped platelets. A lot of research has been dedicated to such processes in Laponite suspensions as gelation 1–3, dispersing 4,5, sedimentation2, dissolution of particles 6. Alongside the fundamental interest, quantitative research of Laponite exfoliation process is important for a range of Laponite applications. Owing to Laponite unique properties, such as ability to form gel at low concentrations, high specific surface area, nonspherical particle shape and presence of adsorption centers on its surface, it is used in nanocomposite gels 7–9, coatings 10, as a stabilizer in suspensions 11 and emulsions 12 and as a carrier for drug delivery 13. For the majority of such applications interaction of Laponite particle surface with the other system components (polymers, drugs, precursors, surface of other materials) is a crucial element for effective design of the target products. Quantitative description of such interactions demands knowledge of the specific surface area of dispersed Laponite, which is determined by the degree of Laponite exfoliation.
Apparently, there are few studies of the Laponite exfoliation process. The study 5 is dedicated to the process of orientation disordering of individual Laponite platelets after their separation from the tactoids. The other work 14 studied the dependence of Laponite particle dimensional parameter on the suspension age using acoustic spectroscopy. The authors made a conclusion that the higher Laponite concentration, the less effective is the process of exfoliation. According to the paper, the tactoids exfoliated completely at the concentration of 1.5% w/w within 4 hours, while at the concentration of 4% w/w the majority of the particles consisted of tactoids of two to three platelets. The authors of the light scattering study 4 determined that Laponite exfoliation for concentration of 0.2% w/w lasted more than 10 hours. However, formation of gel clusters in Laponite suspensions increases scattering light intensity, so decrease of scattering intensity due to exfoliation becomes indistinguishable, thus limiting the use of light scattering method for such study. In the current work we have studied the process of Laponite particles exfoliation by NMR relaxometry, monitoring changes of specific wetted surface area of Laponite suspensions over a period of 3 months.
Materials and Methods
Laponite suspensions were prepared by dispersing Laponite RD powder (BYK Additives & Instruments) in deionized water and subsequent stirring for 24 hours. To prevent dissolution of Laponite in water 6, pH of deionized water was adjusted to 10 by addition of NaOH. To prevent exposure of Laponite to CO2 15, the samples were kept in tightly sealed containers. The prepared suspensions remained homogeneous over the whole period of the study.
1H-NMR relaxometer operating at 13 MHz (Acorn Area particle analyzer, Xigo Nanotools, USA) was used to determine specific area of wetted surface of dispersed objects in suspension. The measured parameters are time of spin-spin (T2) or spin-lattice (T1) relaxation of hydrogen protons. T1 is measured by the inversion recovery method and T2 is measured by the Carr-Purcell-Meiboom-Gill method. According to the two-phase fast-exchange model, the spin relaxation time Ti depends linearly on specific area of wetted surface of the dispersed phase SNMR [16,17]:
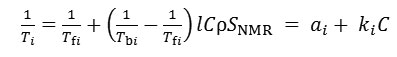
where Tfi is the spin relaxation time of hydrogen protons in free, or bulk, water, Tbi – spin relaxation time of hydrogen protons in water bound to the particle surface, i is 1 (spin-lattice) or 2 (spin-spin), l – thickness of bound water layer (estimated as 1 nm [16,17]), ρ is water density, C – Laponite concentration (solid mass/ water mass),
![]()
In the case of suspensions of exfoliating materials, SNMR is equal to the specific external surface area Sext , which is equal to the full specific area of completely exfoliated particles S less internal surface area Sint enclosed within stacks of particles. Measurement of relaxation times of hydrogen protons T1 and T2 in the suspensions of exfoliating materials allows to make a conclusion about the degree of exfoliation of particles by calculating relative specific surface area:
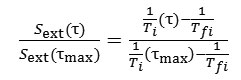
where is the age of the suspension, – the maximum age of the suspension, for which Ti was measured. If at the maximum age of the suspension Laponite particles exfoliated to the fullest possible extent, i.e. , then the ratio and equals exactly to a fraction of individual platelets in the suspension, and ratio – to that of tactoids.
Result and Discussion
Dependence of inverse spin-spin (T2) and spin-lattice (T1) relaxation times of hydrogen protons on Laponite concentration in suspensions of age 92 days is shown in Fig. 1. The intercepts of the dependences with the ordinate axis are equal to the inversed spin relaxation time in bulk water and the slopes are proportional to the specific surface area of the particles in the suspension (Eq. 1). The linearity of the dependences demonstrates that the specific surface area of Laponite particles is constant in the concentration range of 0.5% – 3% w/w. It means that the aged suspensions Laponite tactoids exfoliate to the same extent for the wide concentration range. This conclusion is consistent with the previous findings [16,17] based on the similar experiments. On the contrary, dependence of T2 on Laponite concentration for suspensions of age of 1 day (Fig.1, open symbols) non-linear, meaning that for non-aged suspensions the specific surface area and the degree of particle exfoliation are concentration dependent. By fitting the data for the aged suspensions to the equation (1), the following coefficients were calculated:
a1-1 = Tf1 = 2.78±0.07 s, k1 = 7.6 ± 0.6 s-1, a2-1 = Tf2 = 2.5±0.9 s, k2 = 146 ± 10 s-1.
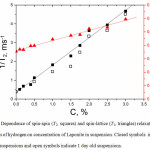 |
Figure 1: Dependence of spin-spin (T2, squares) and spin-lattice (T1, triangles) relaxation times of protons of hydrogen on concentration of Laponite in suspension. Closed symbols indicate 92 days old suspensions and open symbols indicate 1 day old suspensions. |
The sensitivity of the inverse relaxation time to a specific area of Laponite is 19 times larger for spin-spin relaxation (k2/SNMR) compared to spin-lattice (k1/SNMR). Therefore, for examining Laponite exfoliation rates the inverse time spin-spin relaxation was chosen for further calculations. Figure 2 shows a dependence of relative specific area of Laponite in the suspensions, calculated by the equation (2).
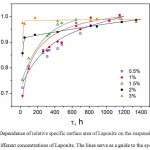 |
Figure 2: Dependence of relative specific surface area of Laponite on the suspension age for different concentrations of Laponite. The lines serve as a guide to the eye. Click here to View Figure |
Table 1: Exfoliation dynamics for Laponite suspensions in water.
| Laponite concentration, % w/w |
0.5 |
1 |
1.5 |
2 |
3 |
| Time, needed for 90% exfoliation, days |
21 |
18 |
11 |
2 |
< 1 |
| Percentage of the internal area enclosed within tactoids after 24 hours of stirring, % |
28% |
31% |
24% |
14% |
3% |
Table 1 shows the characteristic time of exfoliation, when specific external area of the particles in the suspension reaches 90% of the maximum possible specific area; and percentage of the internal area enclosed within tactoids after preparation, which included 24 hours of stirring. The data of table 1 shows that the fraction of tactoids in suspension can be up to 31% after preparation. Surprisingly, for the suspensions with high Laponite concentrations (2% – 3% w/w) the specific surface area was close to the maximum possible area soon after preparation, suggesting much faster exfoliation rate for more concentrated suspensions compared to the less concentrated suspensions (Fig. 2). Such unexpected effect may be caused by the structure of Laponite platelets and the mechanism of their exfoliation. Laponite tactoids are known to exfoliate as follows. When the powder is dispersed in water, sodium ions intercalated between the platelets become hydrated, the initially layered structure weakens and is pushed apart into individual platelets or thinner tactoids by osmotic pressure. At the same time, it is also known that Laponite platelets have negatively charged lateral surface and positively charged edges at pH 10 [18], which are responsible for gelation of Laponite suspensions due to “edge-to-face” interactions resulting in “house-of-cards” gel structure [19]. We suggest that at the initial stages of exfoliation, before gelation, positively charged edges of randomly moving tactoids or platelets provide additional electrostatic attraction force on negatively charged surfaces of neighboring tactoids, facilitating exfoliation of layers already weakened by osmotic pressure. The possibility of such interaction increases with increasing concentration of Laponite. This collective effect would increase the rate of exfoliation for more concentrated suspensions.
Conclusions
In summary, the investigation of Laponite exfoliation process by NMR relaxometry showed prolonged increase of specific wetted surface area of Laponite particles in aqueous suspensions, indicating continuing tactoid disintegration into platelets over extended periods of time – up to three weeks for 1 % w/w suspension. Surprisingly, the suspensions with higher Laponite concentrations demonstrated much faster exfoliation dynamics compared to the less concentrated suspensions. These results should be taken into account in the applications that are based on interactions with exfoliating clay particles surface and depending on the available surface area of the exfoliating materials.
Acknowledgment
The work was financially supported by the Ministry of Education and Science of Russian Federation (RFMEFI57514X0091).
References
- Knaebel, A.; Bellour, M.; Munch, J.-P.; Viasnoff, V.; Lequeux, F.; Harden, J.L. Aging behavior of Laponite clay particle suspensions. Europhys. Lett. 2000, 52, 73-79.
CrossRef - Mongondry, P.; Tassin, J.F.; Nicolai, T. Revised state diagram of Laponite dispersions. J. Colloid Interface Sci. 2005, 283, 397–405.
CrossRef - Shahin, A.; Joshi, Y.M. Physicochemical Effects in Aging Aqueous Laponite Suspensions. Langmuir. 2012, 28, 15674–15686.
CrossRef - Nicolai, T.; Cocard, S. Light Scattering Study of the Dispersion of Laponite. Langmuir. 2000, 16, 8189–8193.
CrossRef - Ramsay, J.D.F.; Swanton, S.W.; Bunce, J. Swelling and dispersion of smectite clay colloids: determination of structure by neutron diffraction and small-angle neutron scattering. J. Chem. Soc. Faraday Trans. 1990, 86, 3919–3926.
CrossRef - Thompson, D.W.; Butterworth, J.T. The nature of laponite and its aqueous dispersions. J. Colloid Interface Sci. 1992, 151, 236–243.
CrossRef - Lorthioir, C.; Khalil, M.; Wintgens, V.; Amiel, C. Segmental Motions of Poly(ethylene glycol) Chains Adsorbed on Laponite Platelets in Clay-Based Hydrogels: A NMR Investigation. Langmuir. 2012, 28, 7859–7871.
CrossRef - Mauroy, H.; Rozynek, Z.; Plivelic, T.S.; Fossum, J.O.; Helgesen, G.; Knudsen, K.D. Oxygen-Controlled Phase Segregation in Poly(N-isopropylacrylamide)/Laponite Nanocomposite Hydrogels. Langmuir. 2013, 29, 371–379.
CrossRef - Sun, K.; Raghavan, S.R. Thermogelling Aqueous Fluids Containing Low Concentrations of Pluronic F127 and Laponite Nanoparticles. Langmuir. 2010, 26, 8015–8020.
CrossRef - Xu, D.; Hodges, C.; Ding, Y.; Biggs, S.; Brooker, A.; York, D. A QCM Study on the Adsorption of Colloidal Laponite at the Solid/Liquid Interface. Langmuir. 2010, 26, 8366–8372.
CrossRef - Loginov, M.; Lebovka, N.; Vorobiev, E. Laponite assisted dispersion of carbon nanotubes in water. J. Colloid Interface Sci. 2012, 365, 127–136.
CrossRef - Yang, Y.; Liu, Z.; Wu, D.; Wu, M.; Tian, Y.; Niu, Z.; Huang, Y. Edge-modified amphiphilic Laponite nano-discs for stabilizing Pickering emulsions. J. Colloid Interface Sci. 2013, 410, 27–32.
CrossRef - Wang, S.; Wu, Y.; Guo, R.; Huang, Y.; Wen, S.; Shen, M.; Wang, J; Shi, X. Laponite Nanodisks as an Efficient Platform for Doxorubicin Delivery to Cancer Cells. Langmuir. 2013, 29, 5030–5036.
CrossRef - Ali, S.; Bandyopadhyay, R. Use of Ultrasound Attenuation Spectroscopy to Determine the Size Distribution of Clay Tactoids in Aqueous Suspensions. Langmuir. 2013, 29, 12663–12669.
CrossRef - Mourchid, A.; Levitz, P. Long-term gelation of laponite aqueous dispersions. Phys. Rev. E. 1998, 57, R4887–R4890.
CrossRef - Duval, F.P.; Porion, P.; Faugère, A.-M.; Van Damme, H. An NMR Investigation of Water Self-Diffusion and Relaxation Rates in Controlled Ionic Strength Laponite Sols and Gels. J. Colloid Interface Sci. 2001, 242, 319–326.
CrossRef - Fripiat, J.; Cases, J.; Francois, M.; Letellier, M. Thermodynamic and microdynamic behavior of water in clay suspensions and gels. J. Colloid Interface Sci. 1982, 89, 378–400.
CrossRef - Tawari, S.L.; Koch, D.L.; Cohen, C. Electrical Double-Layer Effects on the Brownian Diffusivity and Aggregation Rate of Laponite Clay Particles. J. Colloid Interface Sci. 2001, 240, 54–66.
CrossRef - Martin, C.; Pignon, F.; Piau, J.-M.; Magnin, A.; Lindner, P.; Cabane, B. Dissociation of thixotropic clay gels. Phys. Rev. E. 2002, 66, 021401-1 – 021401-11.

This work is licensed under a Creative Commons Attribution 4.0 International License.









