Epoxylitane Сompositions Modification with Using Thermoplastic Polyurethane
K. Syrmanova1, E. Negim2, J. Kaldybekova.1, and A. M. Tuleuov.1
1M. Auezov South-Kazakhstan State University, Shymkent, Kazakhstan
2Faculty of Science and Engineering, University of Wolverhampton, Wolverhampton, UK
Corresponding author email: syrmanova.kulash@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/320101
Article Received on :
Article Accepted on :
Article Published : 19 Feb 2016
This article is devoted for the study of polyurethane modified by epoxylitane coatings solidification processes. The limited ratio of the components in corrosive coating was defined that provides a combination of epoxylitaneresin and thermoplastic polyurethane in presence of polyethylenpolyamine- PEPA. Kinetics of solidification of modified and unmodified epoxylitanecoatings was researched. For the reducinga cost and regulation of coating performance properties was investigated modification of epoxylitane resins with thermoplastic polyurethanes that can be applied for anticorrosion protection of pipelines.
KEYWORDS:oil and gas industry; epoxylitane; coverings excipients; polyurethane; chemical composition of coatings; kinetics of hardening
Download this article as:| Copy the following to cite this article: Syrmanova K, Negim E, Kaldybekova J, Tuleuov A. M. Epoxylitane Сompositions Modification with Using Thermoplastic Polyurethane. Orient J Chem 2016;32(1). |
| Copy the following to cite this URL: Syrmanova K, Negim E, Kaldybekova J, Tuleuov A. M. Epoxylitane Сompositions Modification with Using Thermoplastic Polyurethane. Orient J Chem 2016;32(1). Available from: http://www.orientjchem.org/?p=14069 |
Introduction
The problem of anticorrosion protection of the equipment and facilities of oil refineries (refinery), where more than 30% of the country’s metal reserves acquired are now of paramount importance and requires highly practical solution in the near future.Oil and gas industry in Kazakhstanis one of the main sectors of the Kazakhstan economy, it has a significant impact on the socio-economic development of the country and its regions, in fact, is the engine for the economy of the state, contributes to the development of other sectors of the economy. Kazakhstan has significant reserves of hydrocarbons – 3.3% of world reserves [1]. Currently Kazakhstan continues to consolidate its position as a significant supplier of oil and gas condensate in the world market.
Delivery of oil and gas from the oil wells to the consumers is carried out mainly by pipelines, i.e. this method is the most effective and safe way in the transportation, them over long distances. Pipes are one of the key components in the structure of oil and gas equipment; they integrate individual components into a single technological chain of production complex [2-3]. The increase in hydrocarbon production requires further development of oil and gas transportation infrastructure. It is undeniable that the reliability of pipeline systems is a factor of stability of the economy, as it allows the state to regulate the supply of energy on the external and the internal market. Therefore actually acute losses brought by corrosion. In all industrially – developed countries, they account for 5 – 10% of the national income. Major losses from corrosion – premature failure of steel structures, the manufacturing cost is considerably greater than the cost of the metal used. The second largest item is a set of measures in the corrosion protection [4]. As the analysis of the existing pipelines, one of the main problems is the disparity between the main oil pipelines service life of used insulation materials and life of the pipeline [5].
Emerging recent trends and an increase in processing volume of sulfur and high-sulfur crude oil and gas condensates, and appeared in recent years, the need for protection against corrosion of equipment and installations for the period of the stop and subsequent preservation for a long period require special attention to the fight against corrosion.
In this regard, the ensuring of reliable corrosion protection of main oil pipelines is a very important and urgent task.
Materials and Methods
Significant volumes metal expenses and tough operating conditions oilfield equipment make the problem of increasing the durability of the equipment of one of the central problems of determining the rate of growth and the technical and economic efficiency of extraction and transportation of oil and gas. Delivery of oil and gas from the oil wells to the consumers is provided mainly by pipeline, because this method is the most effective and safe a way for the transportation for long distances.
For the ensuring durability and trouble-free operation of pipelines a system of corrosion protection designed and implemented [5-8]. Usually,for the improving the efficiency of their service, pipelines protect both two fundamentally different ways: anti-corrosion coatings that provide the primary protection of pipelines against corrosion by acting as a barrier that impedes access to pipe water and oxygen in the air, and means of electrochemical that early specific application of polymer coatings for oil and gas the industry is in the variety of simultaneous functions. Therefore, the creating of a coating with the desired combination of properties on the basis of a single material is difficult and in this regard necessary application of different materials in the designs that forms a part of the coating. Directed by changing the design of the coating can be obtained the desired combination of properties on the basis of commercially available materials.
There are a lot of modern anti-corrosion coatings for the enterprise pipelines and trass applications; each of them has its own advantages, disadvantages, applications. A high level of corrosion protection of main oil pipelines is largely determined by the level of technical requirements for pipeline coating and the development of science and technology in the field of corrosion control [5-6]. However, despite all the variety of protective coatings, continuously improve the quality of the coating of pipelines, new insulation materials, new technologies and equipment for coating pipes, to opt for only one coating that would meet all requirements and provide effective protection of pipelines against corrosion under different conditions, is impossible. Due to the development of technologies for the production of coatings for corrosion protection of pipelines, taking into account the conditions of laying and operation is still relevant and it has a great practical importance for the economy of Kazakhstan.
Insulation piping surfaces by thin-layer polymer coating is one of the promising areas in the improving the reliability and efficiency of oil equipment. In products with polymer coating combines the strength and rigidity inherent in metals with chemical resistance, abrasion resistance and a number of other special properties characteristic of polymers. Polymer coatings every year more and more widely used in the oil and gas industry [6-7]. This is explained by the presence in them of a number of valuable properties that allow coatings to perform multiple functions. Polymeric coating protects the surface of the equipment from the corrosive effects of the operational environment, prevent them paraffin and salt, to protect equipment from the waterjet and corrosion-mechanical wear, reduce hydraulic losses, increase the tightness of detachable fixed joints, decrease of metal structures. The coatings can reduce the complexity of repair, reduce the amount of spare parts, alloy steel, nonferrous metals and alloys.
The rational application of a design is determined by the purpose of the coating. Modern sprayed coatings for pipe insulation in field conditions can be divided into two groups: single and double layer. Single-layer coating, in particular: one layer is a protective barrier for the metal. It is self-sufficient for the operating conditions – strong enough impact, abrasion, heat-resistant enough, and so on. The two-layer coating is built on another principle. It protects the inner tube, a relatively thin layer of primer. But it is not sufficiently resistant to shock, especially at low temperatures, is elastic enough so the protective layer itself needs to be protected from the external environment. For that,on the surface is applied the second masticlayer. However, the layers must be firmly interconnected. Single-layer coatings are polyepoxide, but more often – polyurethane or polyurea. Double coatings usually contain layers of different nature. Internal – modified epoxy or polyurethane, outer – the polyurethane-modified polyurethane, at least – epoxy.
Recently the developed coatings that do not have such strict separation function of layers. They are the compositions of the various elastomers, modifiers, fillers and pigments. They often have a set of essential qualities of the constituent components. The technology of applying such composite coatings in multiple layers is monotonous. This speeds up and simplifies the process of forming protective film settings. If the coating has sufficient interlayer adhesion and required resistance to the physical and mechanical influence (sufficient hardness and the need to use an additional outer layer of primer or other elastomers.)
For the preliminary studies the epoxy compositions applied composition that shown in table 1, the development of modified epoxy compositions consisted in the choice of optimum modifier, combining with the epoxy resin and its properties improves.
Table 1: The modified epoxy compositions
|
Resins |
Solvent |
Hardener |
Fillers and pigments |
|
|
Epoxide |
Modifier |
|||
|
EX-20 or EX-16 |
urethane polymer on the base of the polyoxitetramethyleneglycol and 2,4- toluenedenisocyanat |
1,3-dioxyocsalan ethyl cellulose |
polyethylene polyamine |
kaolin, talk, titman dioxide kaolin, talk, titan dioxide |
|
EXR-20, EXR-16 |
polyurethane Vilad 17 |
polyethylene polyamine |
graphite tech.carbon |
|
|
EXR-20, EXR-16 |
polyurethane Vitour |
ethyl cellulose |
polyethylene polyamine |
graphite |
|
EXR-20, EXR-16 |
polyethylenesyloxan liguid |
ethyl cellulose |
polyethylene polyamine |
graphite |
|
EXR-16 |
methylamine phormaldegid resin, bitumen, methylene |
toluene -buthanol |
polyethylene polyamine |
talk, mica |
|
EXR-20 EXR-16 |
bitumen, flotoreagent |
mixture: acetone buthanol, xylene, methyletilkatonebuthil cellulose |
agidolАF-2М |
talk |
|
EXR-20 |
alcylphenolamine resin – oktofor N, rubber |
ethyl cellulose |
agidolAF-2М |
titandioxide, kaolin |
As the hardener agent, aliphatic polyamines are applied. In particular, polyethylene polyamine (PEPA) that can cure an epoxy resin at the room temperature.
As fillers, there were chosen the ingredients that have inert properties for the most aggressive conditions, enhance a chemical resistance, heat resistance, degree of cure epoxy coatings, physical and mechanical properties and adhesion, and also reduce the diffusion of water.
The advantages of applying the epoxy resin as corrosion resistant coatings is a high adhesion, hardness, small curing shrinkage in comparison with other resins, the ability to cure at the room temperature or under heating without the formation of bubbles in the layers of any thickness, high physical-mechanical and electrical properties of the rejected resins as well as high chemical resistance and weather resistance. They can be modified by other film-forming or aligned with them, further enhances their application.
The presence in the epoxy resin of two types of functional groups, hydroxyl and epoxy, allows its solidification different substances capable for the interacting both with epoxy and the hydroxyl groups.
The largest number of epoxy groups contained in the liquid low molecular weight resins such as ED-20, EXR-20. Therefore, these resins after curing the polymer to a greater spatial density mesh than high molecular weight, and therefore with greater strength, rigidity and heat resistance [6-8].
The practical ability of epoxy resins combines with a variety of organic materials opens the approaches to their modification. The purpose of the modification is not only a reduction in consumption and cheaper epoxy coatings but also regulation of certain properties.
Great influences on the properties of epoxy compositions have the nature and amount of filler. Its introduction can significantly change the physical, mechanical, adhesion, dielectric properties, water resistance, shrinkage, and the viability and degree of curing epoxy resins [9].
The processes of solidification modified by polyurethane coatings epoxylitane compositions are studied. For selected compositions necessary to choose fillers and pigments within the parameters of the corrosive environment in which it will be investigated the possibility and efficacy of these compositions. By using the known procedure [8] it was determined the effect on the physical and mechanical properties of the modified polyurethane coatings epoxylitane most frequently used fillers and pigments.
Curing of the epoxy coatings was carried out at the room temperature and under heating. At the room temperature the curing lasted 7 days. At heating till 20,30,40,50,60,80 and 100 °C was carried out in stages, at room temperature overnight, then for 2 hours in an oven at an elevated.
Curing at elevated temperature was carried out in order to study its influence on the degree of cure and adhesion of the coating to the metal.
The depth and completeness of the reaction that characterizes the degree of polymerscuring, determined by the method of extraction or by dissolving and removing the uncured fraction from the polymer.For the study it used a fine powder obtained by pulverizing the film cured at a predetermined temperature. As the extractant acetone wasused. The extraction was carried out in a Soxhlet apparatus.
The extraction time were established by experimentally. Number of the soluble fraction was determined by the formula:
P=(m1-m2)100%/m1
where:
m1 – sample mass before the extraction
m2 – sample mass after the extraction
Thus, for the study of the modified epoxylitane films were applied:
- Epoxylitaneresin- reaction product of diglycidyl ether with epichlorohydrinxylitan [9].
- Polyurethane – product based on polyoxytetramethylene glycol and 2,4-toluenediisocyanate.
- The solvent – 1,3dioxolane.
- Fillers and pigments –kaolin, graphite, mica, talc, silica, titanium.
- Curing -polyethylenpolyamine (PEPA).
Results and Discussion
Technical progress in the development of new technology poses great challenges towards the creation of structural polymeric materials with specific and higher performance through integrated use of raw materials and waste products.
Xylitan uses as raw material for producing a hydroxyl-epoxy compositions of particular interest because of its presence in the composition of the heterocycle and the reactive hydroxyl groups located directly in the cycle, can achieve on its base epoxy oligomers with specific properties. In addition, xylitan rather cheap and available from wastes of pentosancontented plant materials in the form of cotton hulls, straw, corn stalks, reserves are inexhaustible because of the annual sustainability [9].
On the basis of the modified polyurethane epoxylitane coatings (Table 2) compared the properties of the options proposed modification (1-5) and no modified epoxylitane resin (EXR).
For epoxylitane oligomers, inherent in a whole complex of valuable properties of the known epoxy oligomers: a high adhesion, ease of cure, mechanical strength, chemical stability, low shrinkage, etc.
Preparation of the compositions (1-5) was carried out in the following order. The reactor was charged with solvent, polyurethane and after complete dissolution of the charged epoxy resin, filler, pigments and stirred until homogeneous.
Table 2: Properties of the modified options (1-5) and unmodifiedepoxylitane resin (EXR)
|
Components |
Coatings compositions, mass |
|||||
|
modified coatings |
Unmodified coatings |
|||||
|
1 |
2 |
3 |
4 |
5 |
ECR |
|
| Epoxylitane resin EXR -20 |
6.0 |
6.0 |
6.0 |
6.0 |
6.0 |
10.0 |
| Polyurethane |
4.0 |
4.0 |
4.0 |
4.0 |
4.0 |
– |
| Polyethylene polyamine |
1.2 |
1.2 |
1.2 |
1.2 |
1.2 |
2.0 |
| Kaolin |
18.0 |
– |
– |
– |
– |
18.0 |
| Mica |
– |
18.0 |
– |
– |
– |
– |
| Talk |
– |
– |
18.0 |
– |
– |
– |
| Graphite |
– |
– |
– |
18.0 |
– |
– |
| Dioxide titane |
– |
– |
– |
– |
18.0 |
– |
As it is known, epoxy oligomers acquire valuable properties only after curing. Investigation of the kinetics of solidification modified epoxy coatings by extraction with acetone in the Soxhlet device.The kinetics of hardening modified (1-5) and unmodified (EXR) epoxy coating at the 250C for 1,5,10,20,30,40 and 50 days.
Results of research conducted by extraction with acetone and presented at (Figure 1) show that the greatest degree of cure of the films studied there in the first 10 days, in the future degree of cure increases slightly. In the first 10 days of the most intense hardening observed in the film composition №3, and especially №5 (Figure 1) which reaches the level of 76-77% while for films №1, №2 and №4 it is less than 66%. In unmodified film lowest degree of cure and slightly more than in the first 10 days of 67%. After 15 days the difference in the degree of curing is somewhat reduced. Yet, the highest results show the film with an additive as a filler of talc and titanium dioxide.
 |
Figure 1: The dependence of the hardening degree of coatings at 250C and 25% relative humidity in time |
Previous studies were conducted at ambient humidity of 25%. For the determining the effect of humidity on the hardening degree studies were conducted at a relative humidity of 50%. The results of these studies are shown in (Figure 2).
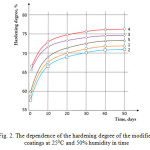 |
Figure 2: The dependence of the hardening degree of the modified coatings at 250C and 50% humidity in time |
From the results the value of the cure degree of corresponding compositions at 50% moisture content differs little from the same degree of cure of the compositions at 25% moisture content (Fig. 2).
Study of the effect of a temperature on the degree of cure has shown that it increases for all the films and at 100°C in the composition №5 (titanium dioxide), its value reaches 89%, whereas the compositions №1, №2, №4 and EXR it in the range of 78-82% (Fig. 3).
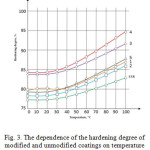 |
Figure 3: The dependence of the hardening degree of the modified and unmodified coatings on temperature |
Conclusion
On the basis of the provided studies it was determined the maximum ratio of the components in the composite coatings, providing the combination of epoxylitane resin and thermoplastic polyurethane in the presence of PEPA. There is 3: 7. It was established the optimum chemical composition of coatings that characterized by a minimum drying time of the films (up to 3 hours). This coating ratio epoxylitane resin and thermoplastic polyurethane in the presence of PEPA is 6: 4, and the expense of PEPA minimal, not more 1,2mass units. Based on a study of the kinetics of curing epoxy-modified coatings found that the degree of cure of the most heavily increased in the first 10 days. At the temperatures above 50 °C increases the curing rate.Thus, for the reducinga cost and regulation of coating performance properties investigated modification of epoxylitane resins with thermoplastic polyurethanes that can be applied for anticorrosion protection of pipelines.
References
- EgorovO.I.;ChigarkinaO.A.;BaimukanovA.S.;Oil and gas complex of Kazakhstan: problems of development and effective functioning. Almaty, 2003. 536.
- KorshakA.A. Pipelines. Ufa,DizaynPoligrafServis,2008. 448.
- KutukovS.E. Information-analytical system of pipelines.Moscow, CIP RIA,2002.324 .
- ErmakovA.V. Challenges of corrosion protection in the industry //Proceedings of the Sixth Interdisciplinary Conference “Corrosion protection of 2015”//.Moscow,2015, . 25-27.
- VereshchaginT.S. Protection against corrosion of the tank farm and oil and gas equipment //International Exhibition and Congress Technologies, equipment and materials for corrosion protection of “Corrosion-2015”//.Saint Petersburg,2015.
- KharisovR.A.;HabirovaA.R.;MustafinF.M.;HabirovR.A. The current state of protection of pipelines against corrosion polymer coatings Ange.Oil and Gas Business, 2005, .3, . 11-27.
- GonikA.A., ManyahinaT.I. Protection of steel oil tanks from corrosion. Moscow,Nedra, 2001, 340.
- RozenfeldI.L.;RubinshteynF.I.;ZhigalovaK.A. Protect metals from corrosion paint by paint-and-lacquer coatings. Moscow, Chemistry, 2007,356.
- SyrmanovaK.K. Polyfunctional reactive oligomers and polymers based on xylitol and xylitan. Composite materials //Proceedings of the International scientific and practical conference “The development of science, education and culture of independent Kazakhstan in the global challenges of our time”, devoted to the 70th anniversary of M.Auezov’ SKSU//.Shymkent, 2013,.7,.141-144.
- Chigorina E.A., Razinov A.L., Kina Y.A.U., Ryabenko V.S. Modification Bituminous Binders Petroleum Resin (Based on C9 Fraction). Orient J Chem2015;31 (4)

This work is licensed under a Creative Commons Attribution 4.0 International License.









