Recent developments in Inorganic polymers: A Review with focus on Si-Al based inorganic polymers
Shrray Srivastava1 and Ravindra Gadhave2
1Department of Polymer Tech., University Institute of Chemical Technology, North Maharashtra University, Jalgaon, India.
2Department of Polymer and Surface Engineering, Institute of Chemical Technology (ICT) Mumbai, India.
Corresponding Author Email: ravi.gadhave3@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310483
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2015
Inorganic polymers are a unique classification of polymers. They contain inorganic atoms in the main chain. Hybrids with organic polymers as well as those chains that contain metals as pendant groups are considered in a special sub-classification as organo-metallic polymers. The networks containing only inorganic elements in main chain are called inorganic polymers. The silicone rubber is the most commercial inorganic polymer. The organo-metallic and inorganic polymers have a different set of applications. The current paper is a review of current applications of polymers with inorganic back-bone networks, especially focusing on Si and Al based inorganic polymeric materials.
KEYWORDS:Inorganic polymers; geopolymers; fire resistant polymers; environmental friendly concrete
Download this article as:| Copy the following to cite this article: Srivastava S, Gadhave R. Furfural Hydrogenation on Alloyed Copper Catalysts With Additives of Ferrosilicium. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Srivastava S, Gadhave R. Furfural Hydrogenation on Alloyed Copper Catalysts With Additives of Ferrosilicium. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13181 |
Introduction
Inorganic polymers, by name, stand for many non-organic monomers/repeating units. Distinguishing between inorganic and organometallic polymers depends on ones opinion. Simply put, inorganic polymers contain only inorganic repeating units, while the organometallics have an alternate organic linkage between the inorganic elements. Metal containing polymers can be further classified as per the position of the metal ion. The metal ions could be in the backbone of the polymer, enmeshed into the chain or as pendent groups to an organic backbone chain. The most common inorganic polymer is polydimethylsiloxane (PDMS), also called silicone rubber. The main interest in this section of polymers arises due to their low temperature elastomeric properties coupled with high temperature stability.
Since the divide between inorganic between the organometallics is very confusing, the polymers can hence be referred to as polymers with non-organic elements. Further, they can be classified as metal in backbone, metal- enmeshed polymers and metal ions in pendent groups [1].
The main reasons for interest in these polymers are their unique property profiles separating them from their organic counterparts. Among them, the main features are [2-4]:
- Number and variety of elements in these polymers
- High abundance of inorganic materials in the earth’s crust
- Stronger bond formation
- High temperature stability
- Tailoring new structures with many variations
Thus, inorganic polymers are an opportunity to expand fundamental knowledge, develop new materials and stimulate new thoughts in polymer chemistry [5].This paper provides an overview of the synthesis; properties and applications of these materials with a special emphasis on the applications of silicate and aluminum based inorganic polymers.
Inorganic polymers
Inorganic polymers of this class are majorly components of soil, mountains and sand. They are used in fire-resistant panels, coatings, flame resistant materials and construction materials. The most widely studied and developed inorganic polymers are silicon based.
Polysiloxanes are the most important inorganic polymers with regard to commercial applications. Their applications range from medical to sealants, water-barriers, cosmetics, etc. PDMS (polydimethylsiloxane) or silicone rubber is the most widely used.
Polysilanes are similar to polysiloxanes in structure. They contain only silicon in the backbone. The applications of polysilanes are based on their property of electron mobility. Their current uses are as photoresists and precursors to other silicon containing materials.
Victor Glukhovsky [6, 7] is believed to be the first researcher to model the geological formation of zeolites, in the 1950s. This discovery of a new class of inorganic materials resulted in wide scientific interest and development of a variety of applications. The most comprehensive work in this field is done by Davidovits [8]. He is also responsible for first coining the term “geopolymer” to alkali-activated alumino-silicates. In 1979, Davidovits founded the Geopolymer Institute in France to increase the scientific and commercial interest in geopolymers and their applications.
The reaction of a solid alumino-silicate with a highly concentrated aqueous alkali hydroxide or silicate solution produces a synthetic alkali alumino-silicate material termed “geopolymer” by
Davidovits [8]. Instead of referring to them as inorganic polymers, geopolymers can more accurately be sub-classified as mineral polymers. Polysialates (polymers containing silicon, aluminum and oxygen in it backbone) were the first polymers to be synthesized. Silicon, aluminum and oxygen are the major constituents of geopolymers. Geopolymers could be made by dissolving alumino-silicate materials in high alkaline environments like sodium hydroxide or potassium hydroxide solution. The products are amorphous to semi-crystalline with good to high mechanical properties [9-19].
Sources of aluminum-silicate include natural minerals and industrial wastes like kaolin, fly-ash, slag from blast furnace, etc. The alkali activation is pH sensitive and NaOH or KOH may be used for this purpose. The polymerization process is however not yet fully understood. The effect of various parameters on the polymerization and the micro-structure has been studied extensively. Geopolymers have successfully been used immobilize toxic metals [16, 19]. Due to its cage structure, geopolymers may even be capable of solidifying radioactive materials.
Synthesis of geopolymers
Geopolymers can be synthesized at room temperature or at slightly elevated temperature by activating aluminosilicate using silicate and aqueous hydroxide solution. The water content is low and hence, the reaction takes place in colloid form. The activator consists of sodium silicate or potassium silicate and NaOH or KOH solution. According to Davidovits [11], the geopolymer consists of Si, Al and O in the backbone. The reaction can be expressed as [16]-
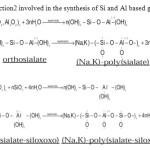 |
Figure 1: Reaction2 involved in the synthesis of Si and Al based geopolymers. |
The SiO 2 in reaction 2 is in monomer or polymer form coming from the silicate solution. The final geopolymer backbone is shown in reaction 2. According to the reaction, activator solution consisting of sodium silicate/ potassium silicate and sodium/potassium hydroxide solution will control the dissolving and charge balance of the reaction system.
Kaolinite [13, 16, 20-23] has been the most widely used material of source for geopolymers. Kaolinite is treated to produce metakaolinite with higher reactivity and activation energy. Potassium polysialate (K-PS) and potassium polysialatedisiloxo (K-PSDS) geopolymer from metakaolinite have shown excellent thermal stability. Sodium silicate and NaOH solution activator system is mainly amorphous and shows layered framework showing that the colloid reaction mainly occurs at flake-like surfaces of metakaolinite particles [24].
Metakaolinvs fly-ash geopolymers
Fly-ash is another source material for aluminosilicate. Fly-ash is a byproduct from thermal power plants. Metakaolin is synthesized from kaolin via various treatment processes. Both the sources have a very different origin. Metakaolin has lesser impurities and hence, is able to give more manufacturing consistency. The particle size [25] may affect the rheology and reaction times but, there is little difference between the reactions.
In contrast to the naturally available kaolin, fly-ash, an industrial waste can also be used as source material for geopolymer synthesis. The chemical composition of fly ash is similar- silicon, aluminum. But, they also contain iron oxides and significant amounts of calcium [26]. The chemical, elemental and phase composition of particles vary from particle to particle and so does their particle size. This non-homogeneity of particles requires more care when working with fly-ash.
Properties of geopolymers
Since 1950s, studies have been carried out in the field of geopolymers with only one application in mind- as cementitious materials. Hence, the properties of faster setting, better compressive strength and durability of geopolymers over traditional cements have been advantageous.
It has been showed that [27] potassium initiated geopolymers show better strength and better setting times than sodium initiated ones. Also, there is a limit to the amounts of NaOH or KOH solution that can be used for polymerization. Excessive amounts are found to have an adverse effect on compressive strength of the material. Similar effect is shown by silicates. However without silicates, the compressive strength is found to be very much lower than with the presence of them.
Geopolymer comopsites
In recent years, geopolymers have been used in composites for their fire-resistant and thermal insulation properties. Lyon [28] studied carbon fiber reinforced geopolymers. Geopolymer composites performed better than organic matrix composites with carbon fibers and glass fibers in flame resistance tests. Even when the geopolymer composites were exposed to more severe environments, they performed better than their organic counterparts.
The effect of length of the fibers was studied by Lin [29] to reinforce a geopolymer matrix. Regardless of the lengths, the filled composites showed pseudo-plastic fracture against the brittle fracture of unfilled geopolymer. In three-point bending tests, a similar pattern was observed where the composites were deformed without full fracture while the geopolymer showed brittle failure. The scanning electron micrographs of the fractured samples showed weak matrix-fiber bonding.
More recently, Bernal [30] studied aluminosilicate fibers and particles filled geopolymers. The composites showed increased thermal stability and reduction in volumetric shrinkage. At higher temperatures, densification of the filler-matrix interface takes place increasing the stiffness. The mechanical performances at high temperatures were also found to be remarkable.
The application of geopolymers in reinforced concrete beams [31] has indicated better performance of geopolymers over organic polymers in adhesion of carbon fibers to reinforced concrete beams.
Geopolymer coatings
Barbosa [33]studied the thermal behavior of potassium polysialate (K-PS) and potassium polysialatedisiloxo(K-PSDS) till about 1400ºC. The geopolymers show excellent thermal stability at temperatures up to 1000ºC. Above 1200ºC, the potassium polysialatedisiloxo becomes porous and friable.
The high thermal properties at such high temperatures are associated with the recrystallization of geopolymer at 1000ºC. Rickard [34-35], used these thermal properties of geopolymers to make heat resistant coatings for metal substrates. The coatings show exceptional balance of lightweight, fire retardant and thermal insulation material. The coatings showed thermal expansion of about 3% at 800ºC. Another observation was the excellent adhesion to the metal substrates.
Future Research
Geopolymers are gaining huge interest in areas where lightweight thermal insulation is required. The study of these materials due to their growing applications across various fields is becoming increasingly important. The research community hence, needs to develop more into this field to produce sustainable as well as futuristic needs from the material.
Also, the long-term study of these materials properties seems a barrier for its growth. Data of severe environments exposed on these materials will benefit further study.
Study of geopolymers as a sustainable replacement to Portland cement is of great importance. The synthesis of geopolymers and their end properties along with durability should be studied.
Conclusion
The history of geopolymers is quite short. Since their birth, they have come a long way in a very short time. The abundance of raw materials in nature and development of commercial applications have secured a large-scale future for them ahead.
The current state of studies coupled with more future work by the research community should help secure a wide acceptance for geopolymers.
However, the fundamental studies need to complete along with large capacity data collection to improve and develop methods for synthesis, sources for raw materials and end-user applications. This will ensure the future of geopolymers.
References
- R.D. Archer, Inorganic and Organometallic Polymers, 2001.
- Stevens M.P., Polymer Chemistry: An Introduction, 2nd ed., Oxford University, 1990,487-508
- Seymour R.B. and Carraher C.E., Polymer Chemistry an Introduction, 3rd ed., Marcel & Dekker, 1992, 381-401
- Rahimi A., Inorganic polymers, polysiloxanes: (1) synthesis and purification of silicon, Proc. of the Int. Sem. on Polym. Sci. and Techno., 3-5 November, 1997, 65-8
- Mark J.E., Allcock H.R., and West R., Inorganic Polymers, Prentice-Hall, 1992, 141-185
- V. D. Glukhovsky: ‘Soil silicates’; 1959, Kiev, GosstroyizdatUkrainy Publishing (in Russian).
- V. D. Glukhovsky: ‘Oil silicates: their properties, technology and manufacturing and fields of application’, DTech.Sc. Thesis, Civil Engineering Institute, Kiev, Ukraine, 1965.
- J. Davidovits: ‘Soft mineralurgy and geopolymers’, Proc. 1st Int. Conf. on Geopolymers, Compiegne, France,1988,1, 19– 23.
- F. Pacheco-Torgal, J. Castro-Gomes and S. Jalali: “Tungsten mine waste geopolymeric binder: preliminary hydration products investigations”, Constr. Building Mater., 2009, 23, 200–209.
- J. Davidovits: J. Therm. Anal., 1991, 37, (8), 1633.
- J. Davidovits: J. Mater. Educ., 1994, 16, (2–3), 91.
- H. Xu and J. S. J. Van Deventer: Int. J. Miner. Process., 2000, 59, (3), 247.
- H. Xu, J. S. J. Van Deventer and G. C. Lukey: Ind. Eng. Chem. Res., 2001, 40, (17), 3749.
- J. W. Phair, J. S. J. Van Deventer and J. D. Smith: Ind. Eng. Chem. Res., 2000, 39, (8), 2925.
- J. W. Phair and J. S. J. Van Deventer: Miner. Eng., 2001, 14, (3), 289.
- J. G. S. Van Jaarsveld, J. S. J. Van Deventer and L. Lorenzen: Miner. Eng., 1997, 10, (7), 659.
- J. G. S. Van Jaarsveld and J. S. J. Van Deventer: Ind. Eng. Chem. Res., 38, (10), 3932.
- J. G. S. Van Jaarsveld and J. S. J. Van Deventer: Chem. Concr. Res., 1999, 29, (8), 1189.
- J. G. S. Van Jaarsveld, J. S. J. Van Deventer and A. Schwartzman: Miner. Eng., 1999, 12, (1), 75.
- F.F.B. Valeria, J.D.M. Kenneth, T. Clelio, Synthesis and characterization of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers, Int. J. Inorg. Mater. 2 2000 309–317.
- H. Xu, J.S.J. van Deventer, Microstructural characterization of geopolymerssynthesised from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HREM, Cement Concrete Res. 2002,32, 1705–1716.
- J.G.S. van Jaarsveld, J.S.J. van Deventer, G.C. Lukey, The effect of composition and temperatureon the properties of fly ash-and kaolinite-based geopolymers, Chem. Eng. J. 2002, 89, 63–73.
- R. Cioffi, L. Maffucci, L. Santoro, Optimization of geopolymer synthesis by calcination and polycondensation of a kaolinitic residue, Resour. Conserv.Recycl. 2003, 40, 27–38.
- Hongling Wang, Haihong Li, Fengyuan Yan, Synthesis and mechanical properties of metakaolinite- geopolymer, Colloids and Surfaces A: Physicochem. Eng. Aspects.2005, 268, 1-6.
- Rahier H, Denayer JF, Van Mele B J Mater Sci.2003, 38,3131
- Cockrell CF, Muter RB, Leonard JW, Anderson RE (1970) Potential for recovering unreacted lime from limestone modified fly ash by agglomerate. Coal Res. Bur.,West Virginia Univ., Morgantown, 267.
- K. Komnitsas, D. Zaharaki and V. Perdikatsis: ‘Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers’, J. Haz. Mater., 2009, 161, 760– 768.
- R. E. Lyon, U. Sorathia, P. N. Balaguru, A. Foden, J. Davidovits and M. Davidovics: Proc. 1st Int. Conf. on Fibre Composites in Infrastructure (ICCI ’96), Tucson, AZ, USA, University of Arizona.1996, 972–981.
- T. Lin, D. Jia, P. He, M. Wang and D. Liang: ‘Effects of fibre length on mechanical properties and fracture behavior of short carbon fibre reinforced geopolymer matrix composites’, Mater. Sci. Eng. A, 2008, 497, 181–185.
- Susan A. Bernal, Julian Bejarano, CristhianGarzon, Ruby Mejia de Gutierrez, Silvio Delvasto, Erich D. Rodriguez,”Performance of refractory aluminosilicate particle/fiber-reinforced geopolymer composites”, Composites: Part B 43,2012, 1919–1928.
- P. N. Balaguru, S. Kurtz and J. Rudolph: ‘Geopolymer for repair and rehabilitation of reinforced c oncrete beams’, Cement Concrete Compos., 1997, 30, 431–443.
- H. Wang, H. Li and F. Yan: ‘Reduction in wear of metakaolinite based geopolymercomposite through filling of PTFE’, Wear, 2005, 258,1562–1566.
- Valeria F.F. Barbosa, Kenneth J.D. MacKenzie, Synthesis and thermal behavior of potassium sialategeopolymer, Materials Letters 2003,57,1477– 1482.
- JadambaaTemuujin, William Rickard a, Melissa Lee, Arie van Riessen, Preparation and thermal properties of fire resistant metakaolin-based geopolymer-type coatings, Journal of Non- Solids.2011, 357, 1399–1404.
- JadambaaTemuujin, AmgalanMinjigmaa, William Rickard, Melissa Lee, Iestyn Williams, Arie van Riessen, Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers, Applied Clay Science. 2009, 46, 265–270.
- Polytetraflouroethylene (PTFE)[32] powder addition into the kaolinite based geopolymer matrix changes the tribological behavior. The wear mode changes from severe to mild due to formation of a soft layer during friction.

This work is licensed under a Creative Commons Attribution 4.0 International License.









