Isolation of alkaloids and anti-tumor activity of the crude methanolic extract of Algerian Cytisus purgans
Ghania Benaiche1*, Noureddine Belattar2, Srecko Trifunnovic3, Nenad Vukovic3, Danijela Todorovic3, Milos Todorovic4, Dejan Baskic4 and Milena Vukić3.
1Department of nature and life sciences, Faculty of Sciences, University of Mohamed Boudiaf, M’sila, Algeria, 2Labortory of applied biochemistry, Department of Biochemistry, Faculty of nature and life Sciences, University of Ferhat Abbas(Sétif 1),Sétif, Algeria, 3Department of Chemistry, Faculty of Sciences, University of Kragujevac, Serbia, 4Faculty of Medical Sciencies, University of Kragujevac, Serbia Corresponding Author Email: ghanobenaiche@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310411
Article Received on :
Article Accepted on :
Article Published : 04 Dec 2015
In this study, two known quinolizidine alkaloids which are sparteine and lupanine were isolated from the methanolic extract of the plant CytisusPurgans of Algerian flora by open column chromatography. These two compounds were identified on the basis of their spectral data (GC/MS, IR, MS, 1H and 13C). The anti-tumor activity of the crude methanolic extract of the aerial parts of the plant was also evaluated invitro against human breast cancer (MDA-MB-231) and human lung cancer (A549) cell lines using MTT assay.
KEYWORDS:Quinolizidine alkaloids; cytisus purgans; Fabaceae; Algerian flora; anti-tumor activity
Download this article as:| Copy the following to cite this article: Benaiche G, Belattar N, Trifunnovic S, Vukovic N, Todorovic D, Todorovic M, Baskic D, Vukić M. Isolation of alkaloids and anti-tumor activity of the crude methanolic extract of Algerian Cytisus purgans. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Benaiche G, Belattar N, Trifunnovic S, Vukovic N, Todorovic D, Todorovic M, Baskic D, Vukić M. Isolation of alkaloids and anti-tumor activity of the crude methanolic extract of Algerian Cytisus purgans. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12911 |
Introduction
The Family of Fabaceae is a cosmopolitan family with about 700 genera and 18000 species, and is one of the most important families, is characterized by a large number of derived traits [1]. This large Family is characterized by an impressive phytochemical diversity. Polyphenols (especially flavonoids and tannins) are common, but from a pharmaceutical perspective various types of alkaloids are probably the most interesting and pharmaceutically relevant groups of compounds [2]. In the genera Genista and Cytisus (both commonly called broom), as well as Laburnum, quinolizidine alkaloids, including cytisine and sparteine, are common [3].
Cytisus is an ornamental genus, it is presented by about 50 species mainly Mediterranean, is native to central and south Europe, Canaries Isles and North Africa. The common name ‘ broom ‘ may have been given to the plant because of its growth habit. Six species represented in Algeria, the more abundant is CytisustrifolusL’her. Other rare species which are Cytisusbalansae Boiss.Et Reut.(Cytisuspurgans), Cytisusfontanesi Spach (Ouarsenis, Bibans…), Cytisussessilifolius L. (Babor) andCytisusboeticus Webb.(North of Oran).CytisusbalansaeBoiss. (Cytisuspurgans) is a rare species of Algeria (Aures, Mahdids, and LellaKhadidja) [4].
Cytisuspurgans is a shrub always more or less ramifies, a trifoliolate and persistent leaves and a yellow flowers, native to central France, north central Spain and Portugal, Algeria and southern Morocco [5].
The antibacterial activity of the MeOH extract of the plant Cytisuspurgans was assayed in vitro by agar disc method against three bacterial species. Cytisuspurgans exhibited antibacterial activity towards allbacteria strains. The maximum antibacterial
activity was shown by Staphylococcus aureus followed by Pseudomonas aeruginosa and Escherichia coli, respectively [6].
Quinolizidine alkaloids represent about 2% of the known alkaloids from plants [7], and most of them were initially noticed as constituents of plants poisonous to humans and livestock. However, accumulated evidence indicates that some of them exhibit potentially useful pharmacological properties like anti-cancer, anti-bacterium, anti-virus, anti-inflammation and pain relief [8].
The sparteine has been used as an oxytocic drug and is of interest because of its antiarrhythmic effect and inhibitory effect of natural killer cell growth [9a and 9b].
Species which contain lupanine must consider potentially toxic, and unsuitable for forage [10].
Many plants of the genus Cytisus are consumed as infusions due to its beneficial effects, for example, Cytisusmultiflorius have terapeutic properties enclosing diuretic, anti-inflammatory, anti-hypertension and antidiabetic effects [11].
Experimental
General
Toluene, ethyl acetate, acetone, diethylamine,dimethyl sulfoxide, methanol, diethyl ether, chloroform, hydrochloric acid, ammonium hydroxide, anhydrous sodium sulfate,Dulbecco’s Modified Eagle’s Medium (DMEM),penicillin, streptomycin, L-glutamine,trypsin and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich Chemie GmbH, Steinheim, Germany.
Silica gel 60 (Merck, 70-230 mesh) was used for column chromatography.Preparative TLC was performed by using silica gel P/UV254 with CaSO4 (Machery-Nagel, Germany, 2 mm layer of adsorbent). Analytical TLC was performed on silica gel (Silica gel 60, layer 0.20 mm, Alugram Sil G, Mashery-Nagel, Germany). Visualization of TLC plates was performed by using UV lamp at 254 nm and 365 nm (VL-4.LC, 365/254, Vilber Lourmat, France), as well as in iodine vapors.
Infrared spectra were run on Thermo Scientific Nicolet 6700 FT-IR spectrometer (4000-400 cm-1). The NMR spectra were recorded on a Bruker Avance III 500 spectrometer,1H NMR at 500.26 MHz and 13C NMR at 125.80 MHz, solvent DMSO d6, TMS internal standard. Chemical shifts were given in d (ppm). Mass spectra were recorded on a Agilent 6890/5973 GC/MS system (Agilent, Santa Clara, CA). GC/MS conditions: HP-5ms column (30 m, id 0.25 mm, film thickness 0.25 mm); split mode of injection (split ratio:40.8:1); injector temperature 270 ºC; pressure 17.18 psi, MSD Transfer Line Heater 280 ºC; flow rate of He 1.3 mL/min, temperature program: initial temperature 150 ºC with increasing of temperature at 10 ºC/min to 290 ºC. Temperature of: MS quadruple detector 150ºC, ion source 230ºC. Mass scan range 35-700 amu at 70 eV. Multiplate reader used for MTT cell viability assay, Zenith 3100(Anthos Labtec Instruments GmbH, Austria).
Plant collection and Identification
Cytisus purgans was collected on June 2013 at Aures Mountains (2328m of altitude) at Khenchela province in the north east of Algeria,and identified by Professor
Mohamed Kaabech, Laboratory of biodiversity and phytogenetic resources, University of Ferhat Abbas, Sétif, Algeria. A voucher specimen is deposited at the herbarium of laboratory of organic chemistry and phytochemistry of University Mohamed Boudiaf of M’sila, Algeria.
Extraction and Isolation of Alkaloids
The air-dried aerial parts of plant were cut into small pieces and were extracted with 99% methanol three times at room temperature. The combined extracts were concentrated, acidified with 5% hydrochloric acid and then, extracted with diethyl ether three times. The aqueous layer was made alkaline with 25% ammonium hydroxide to pH (9-10) and extracted with chloroform six times. The chloroform extracts were combined and dried over anhydrous sodium sulfate and evaporated to dryness in vacuo to give crude alkaloid mixture [12].
Column chromatography was performed on a classic 30 cm long x 4 cm diameter glass column packed with 130 g of silica gel 60 (70-230 mesh). The ethyl acetate solution of 1 g of alkaloid fraction was applied to the top of column by use of a pipette. Elution was started with toluene, then with solvent mixtures with increasing polarities: toluene: ethylacetate: acetone and toluene:acetone:diethylamine. Finally, all 145 fractions (obtained by column chromatography) were monitored by analytical TLC (above mentioned solvent mixtures).
Fraction 141 was rechromatographed on preparative TLC. Mobile phase used for elution was chloroform:methanol:acetone=85:10:5 w/w. After elution and drying of preparative TLC plate, by scraping the layers and additional desorption from methanol, sparteine and lupanine were isolated. Purity of compounds was confirmed on analytical TLC with the
same mobile phase mentioned above. Also, gas chromatographic/mass spectrometric analyses on Agilent 6890/5973 GC/MS system confirmed purity of isolated compounds.
Ant-tumor activity of the crude methanolic alkaloids extract
Preparation of stock solutions
Stock solutions of the alkaloids plant extract was made in dimethyl sulfoxide (DMSO) at a concentration of 100 mg/mL, filtered through a 0.22 mm Millipore filter before use, and diluted by a nutrient medium to various working concentrations, so the final concentration of DMSO in culture medium never exceeded 0.5% (v/v).
Cell Cultures
Human breast cancer (MDA-MB-231) and human lung cancer (A549) cell lines, obtained from Human Tumor Cell Bank (HTB) of the American Type Culture Collection (ATCC), were used in this study. MDA-MB-231 cells were grown in RPMI1640 and A549 cells were grown in DMEM medium. Both media were supplemented with 10 % fetal bovine serum, penicillin/streptomycin and L-glutamine. Cells were maintained in a monolayer culture into the 25 cm2 tissue culture flasks (NunclonTM). The cells were prepared for experiments using trypsinization procedure with trypsin/EDTA.
MTT cell viability assay
Plant alkaloids extract was tested for it effect on viability of two different human tumor cell lines. MDA-MB-231 and A549 cells were seeded in 96-well plates (SarstedtTM) at 3×103 cells in 200 µL of appropriate tissue culture medium per well. Every concentration of examined compound was tested in triplicate, and incubated at 37ºC in a humidified 5% CO2 atmosphere. Control cells contained the appropriated amount of DMSO. Culture medium with corresponding concentration of investigated plant extract, but without cells, was used as a blank.
The viability of cultured cells was determined by assaying the reduction of MTT to formazan. In brief, cells were treated with different dilutions of alkaloids (0.001, 0.005, 0.01, 0.025, 0.05, 0.1, 0.25 and 0.5 mg/mL) or cultivated in the cell culture medium containing the appropriate amount of DMSO (control). After 6h, 24 h and 48h of incubation, the culture medium was removed, and MTT solution was added. After additional 4 h of incubation, medium with MTT was removed and DMSO (150µl/well) was added to dissolve the formazan crystals. The plates were shaken for 10 minutes. Absorbance was measured at 595 nm with a multiplate reader. The results are presented as relative to the control value (untreated cells) [13].
Results and Discussion
The crude MeOH extract of aerial parts of Cytisus purgans was subjected to open column chromatography; two known quinolizidine alkaloids were isolated which are sparteine and lupanine (figure 1).These two compounds were identified on the basis of their spectral data(GC/MS, IR, MS, 1H and 13C).
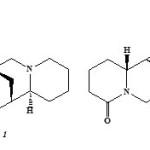 |
Figure 1: Structure of sparteine1 and lupanine2 Click here to View figure |
Spectroscopic data for sparteine1
IR (KBr) n cm-1: 2935, 2816, 2765, 2745 (CH).
1H NMR (DMSO d6, 500 MHz): δ (ppm) 1.26 (2H, m, 5βeq and 8β ax), 1.35 (1H, m, 12αeq),1.38 (1H, m, 5a ax), 1.43 (2H, m, 12β ax and C-H-9), 1.60 (3H, m, 14aeq, 14β ax, 5a ax), 1.68 (4H, m, 13a ax, 13βeq, 4aeq and C-H-6), 1.80 (4H, m, C-H-7, 2β ax, 15a ax and C-H-7), 2.01
(4H, m, 3a ax, 3βeq, 8aeq and C-H-11), 2.19-2.89 (5H, 17a ax, 15βeq, 17βeq, 10aeq, 2aeq).
13C NMR (DMSO d6, 125 MHz): δ (ppm) 22.88 (C-4), 24.86 (C-13), 29.33 (C-3), 29.74 (C-14), 34.31 (C-8), 36.21 (C-5), 36.97 (C-7), 37.11 (C-12), 37.25 (C-9), 53.74 (C-17), 53.78 (C-15), 56.81 (C-2), 61.9 (C-10), 65.21 (C-11), 65.23 (C-6).
MS (70 eV, m/z): 234 (14), 193 (22), 137 (94), 136 (26), 122 (9), 110 (14), 98 (100), 97 (29
Spectroscopic data for lupanine2: Colorless oil, C15H24N2O, Mw=248 g/mol.
IR (KBr) n cm-1: 2931, 2857 and 2765 (CH), 1634 (C=O).
1H NMR (DMSO d6, 500 MHz): δ (ppm) 1.12 (1H, m, 13a ax), 1.15 (1H, ddd, 8b ax, J=10.19 Hz, J=3.98 Hz, J=2.35 Hz), 1.26 (1H, m, 12β ax), 1.38-1.43 (2H, m, 14aeq and 14β ax), 1.45
(1H, m, 5a ax), 1.47 (1H, m, 11 ax), 1.48 (1H, m, 12aeq), 1.51 (1H, m, 4aeq), 1.55 (1H, m, 9 eq), 1.64 (1H, m, 13βeq), 1.70 (1H, m, 4β ax), 1.72 (1H, m, 5βeq), 1.80 (1H, m, 15a ax), 1.86 (1H, dd, J=10.97 Hz, J=3.89 Hz, 17a ax), 1.97 (1H, m, 7 equiv), 2.03 (1H, m, 8aeq), 2.20 (2H, m, 3a ax and 3βeq), 2.44 (1H, m, 10β ax), 2.68 (1H, d, 15βeq, J=9.89 Hz), 2.72 (1H, dd, 17βeq, J=10.97 Hz, J= 3.89 Hz), 3.32 (1H, m, 6ax), 3.70 (1H, m, 10aeq).
13C NMR (DMSO d6, 125 MHz): δ (ppm) 19.74 (C-4), 22.83 (C-13), 24.86 (C-14), 28.53 (C-5), 29.74 (C-7), 33.02 (C-3), 33.77 (C-12), 34.00 (C-9), 38.11 (C-8), 48.52 (C-10), 53.76 (C-17), 56.83 (C-15), 61.08 (C-6), 65.23 (C-11), 168.54 (C=O).
MS (70 eV, m/z): 248 (45), 209 (19), 150 (51), 149 (33), 136 (100), 98 (25).
The IR spectrum of compound 1 showed multiple bands at ~2935 cm-1and at ~2816 cm-1suggested quaternary nitrogen and combination overtones, embodying the C-H stretching the Bohlman’s bands characteristic of quinolizidine-type alkaloids [14].
The peaks at ~1650 cm-1and at ~1400 cm-1indicating the presence of a conjugated carbonyl group and C-N stretching band, respectively [15].
The IR spectrum revealed, also, the presence of an intense peak at 1022 cm-1due tothe C-O stretching band.
The mass spectrum of compound 1 (Mw = 234g/mol) showed a molecular ion [M]+at m/z 234 (23.77 % rel. int.). A base peak at m/z 193 (22.13%) corresponding to [M – 41] +indicating the loss of C3H5 which also showed at m/z 41 (10.65 %) (allylic
cation ). Another base peak at m/z 137 (100%) indicating the cleavage of the compound. The peaks at m/z 110 (18.85 %), m/z 98 (64.75 %) and m/z 55 (9.01 %) corresponding to fragmentation of lupinine ion respectively [16].
It is known also that the fragment at m/z 98 (64.75 %) arises from ring A following the fragmentation pattern of the sparteine-type alkaloids [17].
The mass spectrum of compound 2 (Mw = 248g/mol) showed a molecular ion [M] +at m/z 248 (69.67 %). A base at m/z 247 (50.81 %) corresponding of [M – H] +, another peak at m/z 219 (11.47 %) indicating the loss of 28 units of mass [M – CO] +. The predominant ions at m/z 149 (57.37 %), 136 (100 %), 110 (19.67 %), 98 (20.49 %), 84 (10.65 %), 55 (16.39 %) and m/z 41 (9.83 %) are characteristic of lupanine [18, 19, 20].
Results of NMR spectra were compared with literature data [21, 22].
Anti-tumor Activity
We investigated in vitro anti-tumor activity of the crude methanolic extract of alkaloids of cytisuspurgans on two different cell lines (human breast cancer, MDA-MB-231 and human lung cancer, A 549 cell lines). The cell viability of studied human cancer cells, 6h, 24h and 48h after treatment with increasing concentrations of the plant extract is presented in Figure 2. Numerical data illustrating cell viability were obtained from MTT assays and presented as percentage of control. Tested plant extract is showed no anti-tumor effects toward of both human cancer cell lines. Neither increasing the dose as well as prolongation of the incubation time with the tested plant extract did not lead to a statistically significant increase of anti-tumor effect.
It is known that cytisus species accumulate sparteine-type quinolizidine
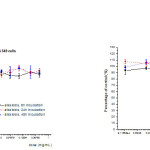 |
Figure 2: Cell viability of MDA-MB 231 and A549 cell lines, 6h, 24h and 48h after treatment different concentrations of crude methanolic extract of alkaloids estimated by MTT assay. Results from three separate experiments are presented as percentage of control (Mean+S.E.). Click here to View figure |
alkaloids as basic constituents [12], this phenomenon is interesting from the view points of chemotaxonomy of leguminous plants and biosynthesis of quinolizidine alkaloids [23].
Quinolizidine alkaloids can be divided into more than six structural groups, although, previous studies demonstrate that matrine-type quinolizidine alkaloids, for example,mediate growth inhibition in different types of cancer cells [13], but sparteine-type quinolizidine alkaloids showed more antibacterian and antiviral effects [24, 25].
To the best of our knowledge, for the first time, we herein report the alkaloid profile of Cytisus purgans of Algerian flora, as well as the anti-tumor activity.
Conclusions
Cytisus purgans is a rare species of Algerian Flora. The phytochemical study of this plant indicate the presence of two known quinolizidine alkaloids: sparteine and lupanine, these two compounds were isolated from the methanolic extract of the plant and identified using different spectroscopic methods.
In the other side the test of the crude methanolic extract of alkaloids against two human cancer cells which are: breast and lung cancer, showed that the plant not exhibited any anti-tumor effect.
Future studies may show more about the benefits of this plant which accumulate quinolizidine alkaloids.
References
- V.H. Heywood, Les plantes à fleurs, Editions Nathan, Paris, (1996).
- B. Bhattacharyya and B. M. Johri , Flowering Plants Taxonomy and phylogeny , Narosa Publishing House , New Delhi , (1998).
- M. Wink, Phytochemistry60, (2003), 3-19.
- G. Lapie and A. Maige, Flore Forestière de L’Algérie, Ed. E. Orlhac, Paris.
- S. Talavera and P. Gibbs, Bottanical Journal of the Linnean Society, 125, Linnean Society of London, London, (1997).
- G., Benaiche, Extraction and GC/MS analysis of major alkaloids found in the family of fabaceae. Academic dissertation, Department of chemistry, Faculty of sciences and Engineer sciences, University of M’sila, Algeria, (2007).
- M. Wink, Quinolizidine alkaloids: biochemistry, metabolism, and function in plants and cell suspension cultures. Planta Med 53, (1987), 509–14.
- Hong G, Liu PX. Advances on alkaloid constituents and their pharmacological effect in plants of Sophora Linn.Zhong Cao Yao (2005); 36:783–8.
- a) M.Raschack, Arzneim. For&. 24, (1974), 753. b) K. Saito, I. Murakoshi, in: A.-u. Rahman (Ed.), Studies in Natural Products Chemistry — Chemistry, Biochemistry and Chemotaxonomy of Lupin Alkaloids in the Leguminosae (2nd edn), Vol. 15, Elsevier, Amsterdam (1995), p. 519
- A. D. Kinghorn, M. A. Selim and S. J. Smolenski, Phytochemistry19, (1980),1705-1710.
- A., Luis, F., Domingues, C., Gil, AP., Duarte, J Med Plants Res, 2009, 3, 886-893
- I. Murakoshi, Y. Yamashita, S. Ohmiya, and H. Otomasu, Phytochemistry25, (1986),521-524.
- Z. L., Chang-Fa Huang, X. Liu and J. Jiang,Basic& Clinical Pharmacology & Toxicology108, (2010), 304–309.
- M. H. Mohamed, Journal of natural products, 56, No. 11, (1993), 1992-2002.
- K. Saito , T. Yoshino , T. Sekine , S. Ohmiya , H. Kubo , H. Otomasu and I. Murakoshi , Phytochemistry28, (1989), 2533-2534.
- I. Murakoshi , E. Kidoguchi , M. Nakamura , J. Haginiwa , S. Ohmiya , K. Higashiyama and H. Otomasu , Phytochemistry20, (1981), 1725-1730.
- V. Christov, H. Dutsheweska, D. Selengha, S. Zhavson and Y. Zhamyansan, J. Nat. Prod.54, (1991), 1413-1415.
- M. Wink, Quinolizidine Alkaloids in Methods in Plant Biochemistry8, Academic Press Limited, London, (1993).
- M. Wink, C. Meibner and L. Witte, Phytochemistry38, (1995), 139-153.
- K. B. Torres , N. R. Quintos , L. L. Barrera Necha and M. Wink , Z. Naturforsch57c , (2002), 243-247.
- B. Jasiewicz, T. Rafałowicz, E. Sikorska, I. Khmelinskii, J. Koput, M. Sikorski, and W. Boczon, Z. Naturforsch. 58 b, (2003), 1133 – 1140.
- B. Jasiewicz, Molecules 13, (2008), 3-10.
- M. Wink, C. Meibner and L. Witte, Phytochemistry 38, (1995), 139-153.
- M. Wink, Z. Naturforsch. 39c, (1984),548 – 552.
- M. Wink, ACS sympser 330, (1987), 524-533.

This work is licensed under a Creative Commons Attribution 4.0 International License.









