DFT study on the adsorption of drug cisplatin onto carbon nanotubes
M. S. Badiee, A. Morsali* and S. A. Beyramabadi
Department of Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran and Research Center for Animal Development Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad 917568, Iran. Corresponding Author Email: almorsali@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310425
Article Received on :
Article Accepted on :
Article Published : 04 Dec 2015
In this work, using quantum mechanics, the interaction of drug cisplatin (CPT) with (5, 5) pristine single wall carbon nanotubes (SWNT) have been studied. All of the calculations have been performed using a hybrid density functional method (B3LYP) in solution phases. Three modes of noncovalent and covalent interactions of cisplatin onto pristine SWNT were investigated. Quantum molecular descriptors and frontier orbital analysis in the drug-nanotube systems were studied. It was found that binding of cisplatin with pristine (CPT/NT) in solution phase is thermodynamically favorable.
KEYWORDS:Cisplatin; Pristine carbon nanotubes; Drug delivery; Quantum molecular descriptors
Download this article as:| Copy the following to cite this article: Badiee M. S, Morsali A, Beyramabadi S. A. DFT study on the adsorption of drug cisplatin onto carbon nanotubes. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Badiee M. S, Morsali A, Beyramabadi S. A. DFT study on the adsorption of drug cisplatin onto carbon nanotubes. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12944 |
Introduction
Cisplatin is a chemotherapy drug, which is used to treat different cancer including testicular, ovarian, bladder, head and neck, and non-small cell lung cancer 1-4.
In recent decades medical and pharmaceutical sciences have witnessed noteworthy advancement in the field of drug design and drug development technologies in the cancer therapy 5-8 . The rapid development of nanoscience has opened new ways in timely and prompt diagnosing of diseases and drug delivery. The use of carbon nanotubes in drug delivery is a new field which is rapidly developing. So far, different systems such as polymers, dendrimers, and liposomes have been used for drug delivery,9-11 but carbon nanotubes provide more effective structures due to high drug loading capacities and good cell penetration qualities12.
Interaction of carbon nanotubes with organic and aromatic molecules through covalent and noncovalent functionalization has been investigated. The main motive behind such investigations has been to improve their solubility and dispersability in aqueous and organic solvents13-19. In spite of extensive use of carbon nanotubes in drug delivery, so far, molecular mechanism of adsorption of cisplatin in water by these nanotubes has not been investigated. In this work, using quantum mechanical methods, the covalent and noncovalent adsorption of cisplatin onto carbon nanotubes was studied.
Computational Details
All of the present calculations have been performed with the B3LYP hybrid density functional level and 6-31G(d,p) basis sets using the GAUSSIAN 03 package.20
The solvent has an important role in chemical reactions explicitly21-27 or implicitly. The implicit effects of the solvent was considered by using the polarized continuum model (PCM). In the PCM method, the molecular cavity is made up of the union of interlocking atomic spheres.
All degrees of freedom for all geometries were optimized in solution (water). The PCM calculations were performed on cisplatin and a pristine armchair (5,5) SWCNT comprising 90 atoms (10 Å). In optimization of the structures, no use has been made of approximations such as the ONIOM28 and in spite of high computational cost, we preferred the results obtained have a good accuracy.
Results and Disscussion
The optimized geometries of cisplatin (CPT) and pristine (NT) single wall carbon nanotubes (SWCNT) in solution phase are shown in Fig. 1. The C–C bond length in pristine (5,5) is calculated to be 1.43 Å, which is in good agreement with the experimental value of 1.44 Å.29
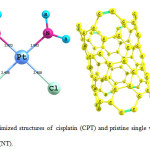 |
Figure 1: Optimized structures of cisplatin (CPT) and pristine single wall carbon nanotubes (NT). Click here to View figure |
Three modes of interaction of CPT onto pristine (5,5) SWCNT were studied. Figures 2-4 present these configurations in solution phase, namely, CPT/NT1-CPT/NT3.
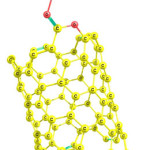 |
Figure 2: Optimized structure of CPT/NT1. Click here to View figure |
The binding energies (ΔE) of cisplatin whit pristine SWCNTs are calculated using the following equation and presented in Table 1:
(ΔE) = EDEX|NT–(ENT+EDEX) (1)
Using the calculated binding energies of CPT/NT1 (covalent adsorption) in Table 1, these energies are negative indicating cisplatin is stabilized by SWCNT surface. Among the 3 configurations, the configuration 1 is the most stable configuration. In this configuration the drug is interacted covalently with the COOH of functionalized SWCNT (covalent adsorption). Using Table 1 this reaction is exothermic (ΔH<0 ) and spontaneous (ΔG<0). In the configuration 2 the drug is interacted with the pristine SWCNT surface through the NH2 group. Configurations 2 and 3 are not thermodynamically favorable.
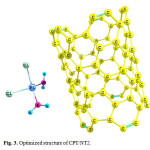 |
Figure 3: Optimized structure of CPT/NT2. Click here to View figure |
In describing stability and chemical reactivity of different systems, quantum molecular descriptors have been used like the chemical potential, the global hardness, the electrophilicity index and etc.
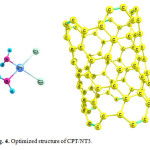 |
Figure 4: Optimized structure of CPT/NT3. Click here to View figure |
The chemical potential (μ) which shows escape tendency of an electron from equilibrium, is defined as follows:
μ = -(I+A)/2 (2)
where I = EHMO is the ionization potential and A = ELUMO is the electron affinity of the molecule.
Table 1: Binding energies of all species (kJmol-1).
|
Species |
ΔE | ΔH | ΔG |
|
CPT /NT1 |
-69.05 |
-62.63 |
-55.43 |
|
CPT /NT2 |
49.37 |
50.99 |
104.03 |
|
CPT /NT3 |
142.66 |
149.80 |
178.02 |
The global hardness (η) shows the resistance of one chemical species against the change in its electronic structure (Equation (3)). Increase in η causes an increase in the stability and a decrease in reactivity.
η = (I-A)/2 (3)
Electrophilicity index (ω) was defined by Parr as follows:30
ω = μ2 |2 η (4)
Tabels 2 represents the values of the quantum molecular descriptors calculated for CPT, NT and CPT/NT1-3 in the solution phases. In this table, besides quantum molecular descriptors, Eg (HOMO-LUMO energy gap) was also presented. Eg notably shows a more stable system.
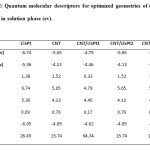 |
Table 2: Quantum molecular descriptors for optimized geometries of different species in solution phase (ev). Click here to View table |
According to the data in Table 2, η, I, and Eg related to the CPT are higher than CPT/NT, showing the stability of the cisplatin decreases in the presence of SWCNT and its reactivity increases. ω of the cisplatin increases in the presence of SWCNT, showing that the cisplatin acts as electron acceptor.
Conclusion
Using density functional theory, the effects of the adsorption of cisplatin onto (5, 5) single wall carbon nanotubes (SWCNT) have been studied in detail in solvent environment. Three possible modes of covalent and noncovalent interaction of cisplatin onto SWCNT (DEX/NT1-3) were investigated. Covalent adsorption (CPT/NT1) is exothermic (ΔH<0) and spontaneous (ΔG<0). Quantum molecular descriptors show that the stability of the cisplatin decreases in the presence of SWCNT and its reactivity increases. The binding energies of the CPT/NT in both phases are negative, so these interactions are energetically favorable.
Acknowledgment
We thank the theoretical research group in research center for animal development applied biology for allocation of computer time.
References
- Marzilli, L. G.; Ano, S. O.; Intini, F. P.; Natile, G. J. Am. Chem. Soc. 1999, 121 , 9133-9142.
- Marcato, P. D.; Fávaro, W. J.; Durán, N., Curr. Cancer Drug Tar. 2014, 14, 458-476.
- Barry, M. A.; Behnke, C. A.; Eastman, A. Biochem. Pharmacol. 1990, 40, 2353-2362;
- Drbohlavova, J.; Chomoucka, J.; Adam, V.; Ryvolova, M.; Eckschlager, T.; Hubalek, J.; Kizek, R. Curr. Drug Metab. 2013, 14 (5), 547-564;
- Tekade, R. K.; Dutta, T.; Tyagi, A.; Bharti, A. C.; Das, B. C.; Jain, N. K. J. drug target. 2008, 16, 758-772.
- Dubey, V.; Mishra, D.; Nahar, M.; Jain, V.; Jain, N. K. Nanomed. Nanotech. Biol. Med. 2010, 6, 590-596.
- Gajbhiye, V.; Jain, N. K. Biomaterials 2011, 32, 6213-6225.
- Nahar, M.; Mishra, D.; Dubey, V.; Jain, N. K. Nanomed. Nanotech. Biol. Med. 2008, 4, 252-261.
- Tomalia, D.; Reyna, L.; Svenson, S. Biochem. Soc. Trans. 2007, 35, 61-66.
- Chonn, A.; Cullis, P. R. Curr. Opin. Biotechnol. 1995, 6, 698-708.
- Allen, T. M.; Cullis, P. R. Science 2004, 303, 1818-1822.
- Prato, M.; Kostarelos, K.; Bianco, A. Acc. Chem. Res. 2007, 41, 60-68.
- Gad, E. A.; Al-Fahemi, J. H.; Khairou, K. S. J. Comput. Theor. Nanosci. 2014, 11, 404-408.
- Yahyaei, H.; Monajjemi, M.; Aghaie, H.; Zare, K. J. Comput. Theor. Nanosci. 2013, 10, 2332-2341.
- Chang, C. M.; Tseng, H. L.; de Leon, A.; Posada-Amarillas, A.; Jalbout, A. F. J. Comput. Theor. Nanosci. 2013, 10, 521-526.
- Lin, Y.; Allard, L. F.; Sun, Y.-P. J. Phys. Chem. B 2004, 108, 3760-3764.
- Star, A.; Liu, Y.; Grant, K.; Ridvan, L.; Stoddart, J. F.; Steuerman, D. W.; Diehl, M. R.; Boukai, A.; Heath, J. R. Macromolecules 2003, 36, 553-560.
- Naghsh, F. Orient. J. Chem. 2015, 31, 465-478.
- Yousefian, Z. Orient. J. Chem. 2014, 30, 1681-1693.
- Frisch, M.; Trucks, G.; Schlegel, H.;Scuseria, G.; Robb, M.;Cheeseman, J.; Montgomery, J.;Vreven, T.;Kudin, K.;Burant, J. Gaussian 03, Revision B.05Gaussian Inc., Pittsburgh, PA,2003.
- Darzi, N.; Morsali, A.; Beyramabadi, S. A. Orient. J. Chem. 2015, 31, 1121-1125.
- Akbari, A.; Hoseinzade, F.; Morsali, A.; Ali Beyramabadi, S. Inorg. Chim. Acta 2013, 394, 423-429.
- Morsali, A.; Hoseinzade, F.; Akbari, A.; Beyramabadi, S. A.; Ghiasi, R J. Solution Chem. 2013, 42, 1902-1911.
- Mohseni, S.; Bakavoli, M.; Morsali, A. Prog. React. Kin. Mec. 2014, 39, 89-102.
- Beyramabadi, S. A.; Eshtiagh-Hosseini, H.; Housaindokht, M. R.; Morsali, A. Organometallics 2007, 27, 72-79.
- Gharib, A.; Morsali, A.; Beyramabadi, S.; Chegini, H.; Ardabili, M. N. Prog. React. Kin. Mec. 2014, 39, 354-364.
- Morsali, A. Int. J. Chem. Kinet. 2015, 47, 73-81.
- Dapprich, S.; Komáromi, I.; Byun, K. S.; Morokuma, K.; Frisch, M. J. J. Mol. Struct. (THEOCHEM) 1999, 461, 1-21.
- Saito, R.; Dresselhaus, G.; Dresselhaus, M. S., Physical properties of carbon nanotubes, Vol. 4; World Scientific. New York, (1998).
- Parr, R. G.; Szentpaly, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922-1924.

This work is licensed under a Creative Commons Attribution 4.0 International License.









