Antioxidant, anti-inflammatory and anti-diarrheal activity of ethanolic extract of medicinal plants grown in Jordan and Palestine
Nooman A. Khalaf, Rajashri R. Naik*, Ashok K. Shakya, Naeem Shalan and Atif Al-Othman
Faculty of Pharmacy and Medical Sciences Al-Ahliyya Amman University, PO Box 263, Amman 19328 Jordan Corresponding Author Email : rajashrinaik4011@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310408
Article Received on :
Article Accepted on :
Article Published : 03 Dec 2015
Herbs like Rosmarinus officinalis (Lamiaceae), Peganum harmala (Nitrariaceae), Teucrium polium (Lamiaceae), Verbena officinalis (Verbenaceae), Artemasia herba-alba (Asteraceae) and Arum palaestinum (Araceae) have been studied for its various biological activities, the extract of fresh aerial parts of plant have not been reported for their antioxidant and anti inflammatory activities. Our aim was to study the antioxidant, anti-inflammatory and antidiarrheal activity of ethanolic extract of fresh aerial parts of the plant. Anti-inflammatory activity was evaluated by carrageenan induced paw edema method, DPPH free radical scavenging activity (antioxidant) and effect of extract on gastro-intestinal tract motility was studied. Phenolic content of Verbena officinalis was higher (652.5 mg GAE %) than the other plant extracts. Whereas antioxidant activity is concerned the Rosmarinus officinalis exhibited highest antioxidant activity (IC50= 6.25 µg/ml), and showed excellent anti-inflammatory (65.5% ) as compared to ascorbic acid (6.14 ± 0.09 µg/ml) and diclofenac sodium (70.1%) respectively. Rosmarinus officinalis leaves extract showed significant inhibition of the gastrointestinal activity (31.3 %) when compared to all the other ethanolic extracts. The ethanolic extract of Arum palaestinum Bioss did not show any significant anti-inflammatory activity nor had any effect on gastrointestinal tract inhibition
KEYWORDS:Antioxidant; anti-inflammatory; Rosmarinus officinalis
Download this article as:| Copy the following to cite this article: Khalaf N. A, Naik R. R, Shakya A. K, Shalan N, Al-Othman A. Antioxidant, anti-inflammatory and anti-diarrheal activity of ethanolic extract of medicinal plants grown in Jordan and Palestine. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Khalaf N. A, Naik R. R, Shakya A. K, Shalan N, Al-Othman A. Antioxidant, anti-inflammatory and anti-diarrheal activity of ethanolic extract of medicinal plants grown in Jordan and Palestine. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12882 |
Introduction
Large numbers of conservationist groups have suggesting that wild medicinal plant be brought to cultivation owing to its therapeutic potential and to meet the growing demand for the herbal medicines. Study conducted among the traditional herbal medicine practitioner of Arab medicine in Jordan, Palestine and the gulf countries suggest that several different plants are being used in herbal therapies. Several medicinal plants and extracted constituents having therapeutic potential are used clinically. Various herbs and spices like Rosmarinus officinalis (1-9), Peganum harmala (10-12), Teucrium polium (13-15), Verbena officinalis (16-20) and Artemasia herba-alba (21) and Arum palaestinum (22-26) have been reported to exhibit a variety of activities including anti-oxidant, analgesic, anticancer, antibacterial, anti-inflammatory, CNS stimulant and depressant activities. The presence of various photochemicals like flavones, iso-flavones, flavanoids, anthocynain, coumarin, lignans, catechins and isocatechins account to the antioxidant activity in plants. Drug formulations possessing antioxidant activity have been reported for the prevention and treatment of certain diseases like atherosclerosis, Alzheimer’s disease, cancer, inflammation, and diabetes (27). The chemotherapeutic, cytotoxic, antibacterial and antifungal potential of R. officinalis, P. harmala, T. polium, V. officinalis, A. herba-alba and A. palaestinum has been reported. To the best of our knowledge the antioxidant and anti-inflammatory activities of fresh aerial plant parts has not been reported so far. The secondary metabolites vary according to the weathering conditions with these criteria in the present study; we report the antioxidant, anti-inflammatory and anti-diarrheal activity of ethanolic extract collected from fresh plant.
Material and Methods
1,1-Diphenyl-2-picryl-hydrazyl (DPPH), loperamide, diclofenac sodium and the other reagents used throughout the experiment were of analytical grade and purchase from Sigma Aldrich Co., St. Louis, USA.
Preparation of Crude Plant Extract
The plant used for the studies were obtained locally or purchased from the local market, north of Jordan Valley and Palestine during Feb-June 2013. Plant material consisting of mature leaves of Rosmarinus officinalis, Peganum harmala, Teucrium polium, Verbena officinalis, Artemasia herba-alba and Arum palaestinum. Washed leaves of different plants were dried using filter paper sheets and then air dried for 2 hours. Leaves were cut and grinded using electrical mixer for 5 minutes. About 200gm of this powdered plant material soaked in ethanol (1 L, 96%) for 24 hours, stirring using magnetic stirrer. The final extracts were filtered using muslin cloth and then through Whatman filter paper No.1 (Whatman Ltd., England). The solvent was evaporated using rotary evaporator at 50 ˚C and the plant extract was stored in refrigerator between 4 to 8 ˚C. For antioxidant activity, stock solutions of crude extracts (5 mg/ml) were prepared by dissolving a known amount of dry extract in 99.5% methanol. The working solutions (1-700 µg/ml) of the extracts were prepared using suitable aliquots of stock solution. For other pharmacological activities the extract was suspended in 10% acacia gum.
Animals
Wistar albino rats (125-160g) and Swiss albino mice (22-23g) were procured from market. All animals were acclimatized at room temperature of 24±2 °C and relative humidity of 55-60%., with free access to food and water for one week. Animal ethics committee guidelines were followed for the experiments on animals. The number of animals used was the minimum necessary to demonstrate the consistent effects of the treatment.
Preliminary test
Determination of Total Phenolic Content28
Total phenolic content (TPC) was estimated following reported method27 with slight modification. Ethanolic solutions of samples (100 µg/mL) were prepared. The sample solutions (1mL) were treated with Folin-Ciocalteau’s reagent (1:10 dilution, 1mL). Then sodium carbonate (25% w/v; 1mL) was mixed with content, vortexed and diluted to 10 ml using distilled water. Sample were vortexed again and the absorbance of resultant solutions were measured at 725 nm using Thermo-Fisher spectrophotometer after incubation at 25 ºC for 1 h. Gallic acid was used as a reference standard for the preparation of calibration curves. Total phenolic content of the samples is reported as mg of gallic acid equivalent (GAE/g).
Antioxidant activity (Free radical scavenging activity)
Oil samples were evaluated using DPPH radical for free radical scavenging activity using the method reported earlier27, 29. Solutions were prepared freshly. DPPH (0.002 g %), different concentration of extract (20 µg/ml – 1280 µg/ml) and ascorbic acid (0.5-25 µg/ml) were prepared in (70% methanol). One ml of DPPH solution was mixed either with different samples of oil (1ml) or ascorbic acid (1ml). These solutions were vortexed at 25°C for 30s and kept aside for 30 min. in dark. Using methanol (70%) as blank at 517nm the instrument spectrophotometer was set at zero. The free radical scavenging activity of residual DPPH against the blank was determined at 517nm using given formula. IC50 (concentration of required to reduce by 50% of the initial quantity of DPPH) are expressed as Mean ± SD (n=3) and determined graphically.
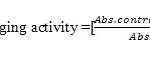
where Abscontrol– is the absorbance at 517 nm DPPH solution and Abssample is the absorbance at 517 nm of DPPH (0.002%) with sample (1mL).
Anti-inflammatory activity
Carrageenan-induced paw edema model was used for studying anti-inflammatory activity30. The animals (albino rats) were divided into groups of four. Plant extracts were given orally (200-300 mg/kg b.wt.) and the paw volume determined plethysmographically (Ugo-Basel, Italy). The control group received equivalent volume of normal saline. The reference group received diclofenac sodium (10 mg/kg b.wt. p.o.). After half an hour the carrageenan (0.1 mL of 1.0% w/v solution) in sterile saline was injected into the sub plantar tissue of the rat’s right hind paw. The paw volume was determined at hourly intervals for 3 hours (0, 1, 2, 3 h). The percent of inhibition of inflammation was determined using formula:
% inhibition = 100 × (Vcon-Vsam)/Vcon ; where ‘Vcon’ represents edema volume in control and ‘Vsam’ edema volume in the group treated with sample.
Effect of oil on gastrointestinal motility29, 31
For the motility studies the mice were fasted overnight with only access to water. The study was performed using the procedure. In which the mice are divided in different groups. Leaves extract samples (100 mg/kg p.o.) containing Tween-80 (0.1 %, 0.1ml) was administered to different animals. Animals of standard groups received Loperamide (8 mg/kg, p.o.) while the control group received Tween 80 (0.1ml, 1%). Charcoal meal (0.3 ml, 10% suspension of charcoal in 1% sodium CMC) was given to animals, 15 minutes after the treatment. Twenty minutes later, animals were sacrificed and small intestine was removed. The total length of intestine (Tt) and distance travelled by the charcoal plug in the intestine (Tc) was measured for each animal. Percentage activity was calculated as:
% inhibition = [1-(Tt/Tc)]*100
Where Tc is the percentage of g.i.t. transit in control group and Tt is the percent of g.i.t. transit in treated group.
Statistical Analysis
GraphPad Prism 5 (San Diego, CA, USA) software was used for statistical analysis. The statistical significance of differences between the groups was analyzed by one-way ANOVA.
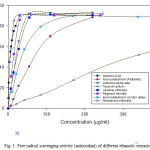 |
Figure 1: Free radical scavenging activity (antioxidant) of different ethanolic extracts. |
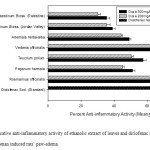 |
Figure 2: Comparative anti-inflammatory activity of ethanolic extract of leaves and diclofenac sodium on carrageenan induced rats’ paw-edema. Click here to View figure |
Results and Discussion
The crude extracts were obtained, ranged from 6.9 to 8.9 g /100 g (w/w). The preliminary phytochemical studies indicate the presence of phenolic compounds in all extracts. The phenolic phenolic content (mg GAE %) were ranged from 22 to 652 mg %. The phenolic content was higher in Verbena officinalis (652 mg GAE%) than the other extract studied. Total phenolic content in Arum palaestinum Bioss (obtained from Palestine and Jordan) were lower than other extracts. Among the 7 extracts and the standard tested for in-vitro antioxidant activity using the DPPH method, the crude ethanolic extracts of Rosmarinus officinalis, Peganum harmala, Teucrium polium, Verbena officinalis, Artemisia herba-alba, Arum palaestinum Bioss (Jordan Velley) and Arum palaestinum Bioss (Palestine) showed free radical scavenging (antioxidant) activity with IC50 values of 6.25 ± 0.12, 15.45 ± 1.01, 321.50 ± 2.6, 23.64 ± 1.73, 20.90 ± 0.95, 36.36 ± 1.25, and 69.75 ± 2.16 μg/ml, respectively (Table 1). The IC50 value for ascorbic acid was 6.14±0.09 μg/ml. The fresh leave extract of Rosmarinus officinalis, Peganum harmala, Verbena officinalis, Artemisia herba-alba and Arum palaestinum Bioss (Jordan Velley) were having excellent antioxidant activity. The results indicate that the antioxidant activity of the crude extract of Rosmarinus officinalis was comparable to ascorbic acid. This also indicates the presence of better antioxidant compounds than ascorbic acid. Further work is required to fractionation of leave extract of Rosmarinus officinalis to obtain the principle constituent. The antioxidant activity of Arum palaestinum Bioss obtained from Palestine was having higher activity than the Jordanian variety. This difference in the activity might be due to the weathering and climatic conditions.
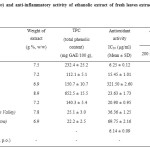 |
Table 1: Antioxidant (in-vitro) and anti-inflammatory activity of ethanolic extract of fresh leaves extract of plants collected from Palestine and Jordan Click here to View table |
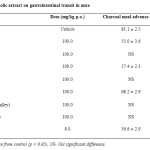 |
Table 2: Effect of different ethanolic extract on gastrointestinal transit in mice Click here to View table |
As far as anti-inflammatory activity fresh leaves extract is concerned, the Rosmarinus officinalis showed excellent anti-inflammatory activity against carrageenan induced paw edema (65.5%) at 200mg/kg b.wt. dose. Significant anti-inflammatory activity were observed by Verbena officinalis (63.6%), Teucrium polium (50.5%), Peganum harmala (45.1%) and Artemisia herba-alba (44.2%) at 200mg/kg b.wt. dose. Significant increase in the anti-inflammatory activity was observed at higher dose of extract (300mg/kg b.wt.). The leave extracts of Arum palaestinum Bioss did not produce significant anti-inflammatory activity.
Significant inhibition in gastrointestinal activity was observed with the fresh leave extract of Rosmarinus officinalis, Teucrium polium and Artemisia herba-alba, which might be due to their direct effect. The maximum percent inhibition (31.3%) was observed with leaves extract of Rosmarinus officinalis. The percent inhibition of g.i.t. transit for loperamide was 54.3%. Other extracts did not affect the gastrointestinal transit of charcoal meal (at 100 mg/kg b.wt. dose).
Conclusion
In the present study on some of the most popular and traditionally used Jordanian plants (Rosmarinus officinalis, Peganum harmala, Teucrium polium, Verbena officinalis, Artemasia herba-alba and Arum palaestinum), it may be concluded that the extract Rosmarinus officinalis leaves showed higher antioxidant activity associated with appreciable anti-inflammatory activity. It also inhibits significantly the gastrointestinal motility in comparison to the other extracts. A. plalestinium did not exhibit anti-inflammatory and had no effect on intestine.
Acknowledgement
Author wish to thank Dean, Faculty of Pharmacy and Medical Sciences and Dean, Higher Education & Scientific Research, Al-Ahliyya Amman University, Amman, Jordan for providing necessary facilities.
References
- Lottenberg, A.M.; Torres, R.P., Mancini-Filho, J. Nutr. Metab. (Lond). 2013, 10(1), 19.
- Ahmed, S.B.; Sghaier, R.M.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaali, I. Nat. Prod. Res., 2011, 25(12) 1195-201.
- Barni, M.V.; Carlini, M.J.; Cafferata, E.G.; Puricelli, L.; Moreno, S. Oncol. Rep. 2012, 27(4), 1041-8.
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. J. Agric. Food Chem. 2012 60(38), 9603-608.
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Food Chem , 2013, 136(1), 120-129.
- El-Omri, A.; Han, J.; Ben-Abdrabbah, M.; Isoda, H. Cytotechnology, 2012, 64(3), 231-40.
- Vicente, G.; Martín, D.; García-Risco M.R.; Fornari, T.; Reglero, G. J. Oleo. Sci. 2012, 61(12), 689-97.
- Lucarini, R.; Bernardes, W.A.; Ferreira, D.S.; Tozatti, M.G.; Furtado, R.; Bastos, J.K. ; Pauletti, P.M.; Januário, A.H.; Silva, M.L.; Cunha, W.R. Pharm. Biol. 2013, 51(9), 1087-1090.
- Tsai, T.H.; Chuang, L.T.; Lien, T.J.; Liing, Y.R.; Chen, W.Y.; Tsai, P.J. J. Med. Food. 2013, 16(4), 324-33.
- Hamden, K.; Carreau, S.; Ayadi, F.; Masmoudi, H.; El-Feki, A. Biomed. Environ. Sci. 2013, 22(5), 381-387.
- Soliman, A.M.; Abu-El-Zahab, H.S.; Alswiai, G.A. Asian Pac. J. Trop. Med. 2013, 6(4), 285-95.
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Food Chem. Toxicol. 2013, 55, 202-8.
- D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; D’Angelo, G.; Galasso, S.; Monaco, P. Nat. Prod. Res. 2013, 27(4-5):356-63.
- Tepe, B.; Degerli, S.; Arslan, S.; Malatyali, E.; Sarikurkcu, C. Fitoterapia. 2011, 82(2), 237-46. doi: 10.1016/j.fitote.2010.10.006. Epub 2010 Oct 19.
- Mehrabani, D.; Rezaee, A.; Azarpira, N.; Fattahi, M.R.; Amini, M.; Tanideh, N.; Panjehshahin, M.R.; Saberi-Firouzi, M. Saudi Med. J. 2009, 30(4):494-9.
- De Martino, L.; De Feo, V.; Fratianni, F.; Nazzaro, F. Nat. Prod. Commun. 2009, 4(12), 1741-50.
- Casanova, E.; García-Mina, J.M.; Calvo, M.I. Plant Foods Hum. Nutr. 2008, 63(3), 93-97.
- Speroni, E.; Cervellati, R.; Costa, S.; Guerra, M.C.; Utan, A.; Govoni, P.; Berger, A.; Müller, A.; Stuppner, H. Planta Med. 2007, 73(3):227-35.
- Calvo, M.I. J. Ethnopharmacol. 2006, 107(3), 380-2.
- Deepak, M.; Handa, S.S. Phytother. Res. 2000, 14(6), 463-5.
- Laid M.; Hegazy M.E.F.; Ahmed A.A. Phytochem. lett. 2008, 1, 85-88.
- Husein, A.I.; Ali-Shtayeh, M.S.; Jondi, W.J.; Zatar, N.A.; Abu-Reidah, I.M.; Jamous, R.M. Pharm. Biol. 2014, 52(10), 1249-55.
- Ali-Shtayeh, M.S.; Jamous, R.M.; Jamous, R.M. Complement. Ther. Clin. Pract. 2011, 17(4), 235-40.
- Al-Mustafa, A.H.; Al-Thunibat, O.Y. Pak. J. Biol. Sci. 2008, 11(3), 351-8.
- El-Desouky, S.K.; Kim, K.H.; Ryu, S.Y.; Eweas, A.F.; Gamal-Eldeen, A.M.; Kim, Y.K. Arch. Pharm. Res. 2007, 30(8), 927-31.
- Afifi, F.U.; Khalil, E.; Abdalla, S. J. Ethnopharmacol. 1999, 65(2), 173-7.
- El-Agbar, Z.A.; Shakya, A.K.; Khalaf, N.A.; Al-Haroon, M. Turk. J. Biol. 2008; 32, 193-196.
- Gutfinger, T. J. Am. Oil Chem. Soc. 1981, 58, 966-968.
- Naik, R. R.; Shakya, A. K., Khalaf, N. A.; Abuhamdah, S.; Oriquat, G.A.; Maraqa, A. Jordan J. Pharm. Sci. 2015, 8(3), 181-193.
- Winter, C.A.; Risley, E.A.; Nuss, G.W. J. Pharmacol. Exp. Ther., 1963, 141, 369-76.
- Borrelli, F., Capasso, F., Capasso, R., Ascione, V., Aviello, G., Longo, R., and Izzo, A.A., Br. J. Pharmacol. 2006; 148, 553-60.

This work is licensed under a Creative Commons Attribution 4.0 International License.









