Using Modified Sorbents for Reducing Negative Impact of Oil-Containing Industrial Wastes on Natural and Artificial Waterways
Ljudmila Anatolievna Marchenko, Gennady Ivanovich Kasyanov, Artem Andreevich Marchenko and Maria Vjasheslavovna Nizhivenko
Kuban State Technological University, 2, Moskovskaya st., Krasnodar, 350072 Russian Federation
DOI : http://dx.doi.org/10.13005/ojc/310342
Article Received on :
Article Accepted on :
Article Published : 12 Sep 2015
The study is aimed at developing and using co-precipitated hydroxides (CPH) of aluminum and magnesium as sorbents, in order to reduce the negative impact of oil-containing industrial wastes on natural and artificial waterways. A new method of synthesizing a modified sorbent has been developed, featuring high sorption capacity to a wide range of pollutants in low-acid, neutral, and low-alkaline environments, able to extract complex compounds. A possibility to use this sorbent has been shown, and its sorption capacity has been studied. Sorption parameters have been defined. It has been shown that the value of the maximum achievable concentration efficiency in extracting the said ions on a co-precipitated sorbent is about ten times higher than the corresponding values that characterize sorption on analogous sorbents. In performing analytical studies, standard methods were used, as well as modern methods of physical and chemical analysis: x-ray phase, x-ray fluorescent, atomic absorption, spectral, chemical, thin layer chromatography, and chromato-mass-spectrometry. The specific surface of the samples was defined by the temperature of nitrogen absorption, using the chromatographic method, followed by processing the obtained results using the Brunauer-Emmett-Teller method. Porosity was defined using mercury porometry. The performed research has made it possible to obtain new highly efficient sorbents and assess their economic efficiency. The performed research will make it possible to resolve ecological and social problems by preventing the damage caused by environmental pollution with anthropogenous wastes.
KEYWORDS:sorption capacity; modified sorbents; filtration; heavy metal ions; oil-containing water; treating; co-precipitation; impurities; toxicity
Download this article as:| Copy the following to cite this article: Marchenko L. A, Kasyanov G. I, Marchenko A. A, Nizhivenko M. V. Using Modified Sorbents for Reducing Negative Impact of Oil-Containing Industrial Wastes on Natural and Artificial Waterways. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Marchenko L. A, Kasyanov G. I, Marchenko A. A, Nizhivenko M. V. Using Modified Sorbents for Reducing Negative Impact of Oil-Containing Industrial Wastes on Natural and Artificial Waterways. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10817 |
Introduction
Treating industrial wastewaters is closely related to environment protection, and is today’s urgent problem. One of the main tasks of preserving the ecosystem of the Kuban River is decreasing the anthropogenous load, and decreasing discharge of insufficiently treated wastes, especially of Krasnodar and major cities in the Krasnodar territory. So the Northern Industrial Area of Krasnodar contains an industrial complex that annually discharges over 3 mln. m³ of insufficiently treated wastes containing highly toxic substances. The main sources of pollution of waterways in the Krasnodar territory are agricultural, chemical, petrochemical and construction enterprises. An urgent problem of urban sewage treatment facilities are “salvo” discharges of highly toxic substances (petroleum products, chlorinated hydrocarbons, phenols, heavy metals), which lead to malfunctions in the treatment facilities.
Today, there still exists a necessity to develop the legislation in the area of ecological safety and its regulation. By the resolution of the Legislative Assembly of the KrasnodarTerritory dated 24.05.2012 No. 3258-P a concept was approved for treating industrial and consumption wastes in the Krasnodar territory till 2020.
The strategic goal of treating wastes is creating an efficient waste management system in the territory, which would ensure ecological safety and create conditions in which the companies in the Krasnodar territory will be advantageous to use alternative technologies.
According to the data of monitoring in 2013, the water in rivers in the Kuban basin belonged to third and fourth quality classes, “polluted” and “very polluted” [1].
Increased concentrations of heavy metals were found in the KubanRiver, namely: the content of copper compounds amounted to 18 MPC (maximum permissible concentration), for iron, the total was 4 MPC, for phenols – 5 MPC, and for zinc – 11 MPC.
The average concentration of oil products in 2013 in the delta of the KubanRiver amounted to 0.06 − 0.09 mg/dm³. The maximum value was 0.10 mg/dm³ (2 MPC). The potential sources of oil products’ ingress into the waterways are surface rain waters and small boats.
Dissolved mercury in the delta of the Kuban River was monitored only in the area of Temryuk. In 2013, traces of mercury were detected in 6 samples out of 24. Presumably, mercury enters the Kuban River by transit from upstream and with surface runoff.
Also, the Krasnodar territory is one of the oldest oil-producing regions of Russia. It has 98 deposits of hydrocarbons (63 oil, 24 oil-and-gas, and 11 oil-and-gas condensate) with the total oil productive capacity in the category of 57.76 mln. tons [1].
In order to determine hazards to the environment, sludge hazard classes were calculated. Allocating sludge to a particular environment hazard class is possible on the basis of indicator (K), which characterizes the level of environment hazard caused by the component of the wastes. The waste component hazard (Ki) is calculated as a function of its concentration in the wastewater (Ci) with a coefficient of hazard to the environment (Wi).
The unit of measurement of wastes’ hazard coefficient is conditionally accepted as mg/kg. The results of calculating the environmental hazard indicator of oil sludge from an oil sludge tank (K) are shown in Table 1.
Table 1: The results of calculating the environmental hazard indicator of oil sludge from an oil sludge tank (K).
|
Item No. |
Components of the waste |
Wi mg/kg |
Ci mg/kg |
Ki |
|
1 |
Iron oxides |
7,413.1 |
19,718.75 |
2.67 |
|
2 |
Manganese |
18,197 |
449 |
0.025 |
|
3 |
Lead |
2,138 |
179.2 |
0.084 |
|
4 |
Copper |
10,000 |
622 |
0.23 |
|
5 |
Chrome |
5,754 |
132.5 |
0.062 |
|
6 |
Petrochemicals |
2,951.2 |
822,900 |
278.8 |
|
7 |
Total |
282 |
The indicator of waste’s environmental hazards K=282. Basing on the value of the environmental hazard indicator in the table “Criteria for classifying hazardous wastes by environmental hazard”, its hazard class was defined: 103>282>102; therefore oil sludge from oil sludge tanks belongs to the 3d hazard class; and therefore it can be used as a secondary raw material [2].
Wastewater was discharged into natural waterways of the Krasnodar territory in 2013 by 238 respondents with access to discharging wastewater into natural waterways. In 2013, over 2,677.86 million m³ of wastewater were discharged into the natural waterways of the Krasnodar territory (in 2012 – 3,105.91 million m³). Out of these, normatively clean (without the need for treatment) – 1,715.29 million m³, and those requiring treatment – 962.57 million m³. Discharge was: polluted (without treatment water) – 712.75 million m³, insufficiently treated – 126.56 million m³, and normatively purified at the treatment installations – 123.25 million m³ [1].
The share of each pollutant in the overall pollution of natural waterways in the Territory has certain negative consequences. For the Krasnodar region and Russia as a whole, the problem of industrial and consumption wastes management is a priority.
Efficient treatment of wastewater from industrial enterprises has been successfully dealt with by Russian and foreign specialists for a long time [3-5]. However, there is no consensus on this issue, unfortunately. The task of reducing the level of influence of the industrial wastewater remains urgent.
In general, in the existing situation, new wastewater treatment technologies should be developed, and existing technologies should be upgraded. One of the promising methods of treating wastewater is sorption with the use of effective efficient sorbents.
Methodology
For the synthesis of co-precipitated hydroxides of magnesium and aluminum we used 1.0 n solutions of magnesium and aluminum nitrates, mixed in the molar ratio Mg(NO3)2:Al(NO3)3 4:1 (e.g., 40 g and 10 g) that were considered the best by the results of experimental data. Vigorously stirred, they were poured into five volumes of water, while adding a 1 n. solution of sodium hydroxide.
The solution was mixed with a magnetic stirrer, the speed of the reactants fusion was 2-3 ml per minute with controlling the pH of precipitation at 8.9. The precipitation was then washed with distilled water using the method of decantation, after which it was pressed and subjected to consequent modification with polyhexamethylenguanidine and 1,8-dioxynaphthalene-3,6-disulfonic acid, with the pH of modification being 8. It was found that as early as after the single treatment of CPH (co-precipitated hydroxide) with 10 ml of a 0.1% solution of PHMG, complete coverage of the surface of the CPH with PHMG macromolecules was achieved, with achieving full sorption capacity of the sorbent. After the modification, the materials were granulated by drying at 120 °C. The main fraction of the granulated materials was 2.5-3 mm particles.
Linear polyhexamethylenguanidine was chosen as polymeric polyamine:
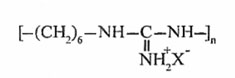
where X is an anion of organic or organic acid; n = 30-90.
Results and Discussion
Low soluble non-organic compounds may be considered as the basis for synthesizing materials capable of absorbing a wide range of ions from aqueous solutions. Table 2 shows the sorbents based on oxides and hydroxides of metals that can be used as sorbents [6]. The data in Table 2 show that there is a wide range of tasks resolved with the use of non-organic ion-exchange materials.
Let us note the two main advantages of non-organic ion-exchange materials: these materials feature much higher radiation stability, as compared to ion-exchange resins, and in the number of cases their thermal stability is higher than the same characteristic for ion-exchange resins [7, 8, 9]. Therefore, reviewing selective properties of non-organic sorbents shows that this material may be considered as an efficient addition to modern methods of ion separation.
The main criterion for selecting non-organic oxides as matrices for sorbent synthesis is their availability.
Unlike polymeric organic matrices, non-organic sorbents do not feature swelling; therefore, no special treatment of sorbents is required before the process of sorption concentration followed by determination [7].
The absence of the phenomenon of sorbent particles abrasion in the process of intensive stirring in static mode and compaction during sorption in the dynamic mode is defined by mechanical strength of the particles [9].
From the literature it is known that another criteria for selecting non-organic sorbents as matrices for synthesis of co-precipitated oxides (CPO) is chemical activity of the their surface due to the availability of many surface hydroxyl groups, which are used for fixing organic reactants.
Table 2: Non-organic sorbents for selective adsorption of ions
|
Sorbate |
Non-organic sorbents |
Assessment of selectivity features of the sorbent and conditions of its use |
Literary source |
|
|
Co2+ |
Hydrated SnO2 |
Maximum sorption at the pH observed at the start of Co hydroxide precipitation |
[6] |
|
|
SeO32-
|
Hydrated ZrO2 applicated with ions
SeO32- |
Selective sorption, pH = 1÷2. Separation from Te(IV) at pH=2 ÷10 |
[10-11] |
|
|
Сl–, Вr–, I– |
Hydrated SnO2 |
Selectivity row Сl–< Вr–<I– |
[12] |
|
|
Hydrated ZrO2, A1(Oh)3 |
Separation of Сl–,Вr–, I– ions |
[13] |
||
| Mo(VI) |
Hydrated Nb2O5 applicated with ions of Mo(VI) |
The sorbent is selective for Mo(VI) ions |
[14] |
|
|
W(VI) |
Hydrated ZrO2 |
Selectivity row МоО42->WO42->VO3–>>ReO4–, pH<9 |
[15] |
|
|
Fe(III) |
MnО2 applied to silica gel |
Adsorption of the colloidal forms of Fe(III) |
[16] |
|
|
Fe2+, Fe3+ |
Hydrated Ni(OH)2+NiOOH |
Removing traces of Fe(II, III) from Ni salts solutions |
[17] |
|
|
Co2+ |
Hydrated NiOOH |
Removing traces of Со from Ni salts solutions |
[18-19] |
|
|
Cu(II) |
Hydrated FeS |
Removal from shaft waters |
[20] |
|
|
Hydrated No |
Sorption from a pH>12 ammonia solution |
[21] |
||
|
Sorbent on magnesium-iron sludges |
Removal of Со2+, Cu2+, Ni2+ from industrial wastes |
[22] |
||
|
Zn2+ |
MgO, Mg(OH)2 |
Removal of Zn2+ and other heavy metals from industrial wastes |
[23] |
|
The nature of hydroxides of metals has been studied in many papers; however, there no consensus on this issue has been reached until now.
For a long time, there have been different views on the nature of hydroxides in the literature. Various approaches to explaining the nature of hydroxides were due to the fact that the problem of the nature of water in them was solved in different ways.
One of the most generalized research in the area of metal hydroxides chemistry was research by V. P. Chaly [24], who studied regularities of formation, the composition, the structure and the properties of precipitated hydroxides of 26 metals and 26 hydroxide systems with one, two, three, four and five components, in relation to obtaining important inorganic materials, namely, sorbents, catalysts, and ferrites.
There is a method of obtaining sorbent for wastewater treatment that includes alkaline co-precipitation of hydroxides of metals from a solution of magnesium and aluminum salts in the proportion of 2:1 at pH 9.6-10.0, formation of precipitation, granulation, and drying the granules with a size of not more than 2.5-3 mm, at 115-120°C (patent RU No. 2343120) [2].
The known method ensures obtaining an oxide solid phase that can be used as a carrying agent. However the disadvantage of this method is using an expensive material, silica gel, as a carrying agent for the sorbent.
There is a method of obtaining a modified sorbent for wastewater treatment, including consecutive modification of oxide carrying agent with polyhexamethylenguanidine (PHMG) and 1,8-dioctonaphtalene-3.6-disulphonin acid (chromotropic acid) – (patent RU 2380152) [25].
The disadvantage of this method is obtaining a sorbent with insufficiently high sorption capacity for ions of heavy metals; moreover, the state of the sorbent (operating appearance) is not indicated.
We aim at developing a method of obtaining a modified sorbent for wastewater treatment, and expanding the range of methods of similar nature.
The technical result is creating modified sorbents with high sorption capacity for a wide range of aliovalent ions in low-acidic, neutral and low-alkaline environments, with the ability to extract elements.
The technical result is achieved due to the fact that in the method for producing a modified sorbent for wastewater treatment, including sequential modification of the oxide carrying agent with polyhexamethylenguanidine and 1.8-dioxynaphthalene-3,6-disulphonic acid, co-precipitated hydroxides of magnesium and aluminum were used as the oxide carrying agent. Such hydroxides had been obtained by co-precipitation of salts magnesium and aluminum in the alkaline environment in the ratio of 4:1, at pH of 8.9; pH being 8-9 at the stage of modification, and after the modification, granulation was performed by drying, with obtaining 2.5-3 mm fractions at 115 to 120°C. Magnesium hydroxide’s ability to condensate stepwise with formation of mechanically stable structures made it possible to eliminate the phase of adding binders.
Here, precipitation pH was controlled within 8.9. Then, the obtained co-precipitated hydroxides of metals were consequently modified with polyhexamethylenguanidine and 1,8-dioxynaphthalene-3,6-disulfonic acid, the pH of modification being 8-9. After modification, the materials were granulated by drying at 115-120°C. The main fraction of the granulated materials was 2.5-3 mm.
In choosing the sorbent, technical requirements were taken into account, such as: specific fractional composition, mechanical strength, chemical stability of the material to filtered water. The mechanical strength of the filtering materials is characterized by wearability and grindability. The material with grindability not exceeding 4%, and wearability ща 0.5% is considered mechanically strong. It is established that grindability of the sorbent synthesized is 1.42 %, and abrasion is 0.31 %. Stability of the sorbent in different reaction media was studied. The results are shown in Table 3.
Table 3: Indicators of filtering material’s chemical resistance
|
Medium |
Oxidizability, mg/dm³ |
Oxidizability, mg/dm³ maximum allowable parameters |
|
NaOH |
9.37 |
10 |
|
HCl |
9.68 |
10 |
|
NaCl |
8.49 |
10 |
Thus, the material obtained meets the regulatory requirements by all parameters.
The most common characteristic of the sorbent is its specific surface defined by the total volume and pores size.
The specific surface area was determined by low temperature nitrogen adsorption using the chromatographic method of analysis with subsequent data processing according to the Brunauer–Emmett–Teller surface area equation. Adsorption and structural characteristics of the sample at 120°C are shown in Table 4.
Table 4: Adsorption and structural characteristics of the samples
|
Sample |
Specific surface, m²/g |
Pore volume, cm³/g |
Effective pore radius, A0 |
|
|
Total |
Small |
|||
|
sorbent |
160 |
0.83 |
0.100 |
50-75, 1,150-1,400 |
The obtained data show that the examined sample meets requirements to sorption materials.
The main criteria for choosing PHMG for creating an intermediate layer between the surface of the inorganic oxide and the complex-forming reactant is convenient spatial arrangement of the primary amino groups in the guanidine group, which creates favorable conditions for formation of hydrogen bonds and makes it possible to have primary amino groups that are not bonded to the surface hydroxyl groups.
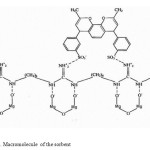 |
Figure 1: Macromolecule of the sorbent Click here to View figure |
 
PHMG is a polyelectrolyte, a stronger organic base (pKa~13.5) as compared to polyethyleneimine (pKa~10). Therefore, in a wide range of pH solutions, amino-groups of PHMG are protonated, which leads to forming hydrogen bonds with hydroxyl groups of the surface of CPO, as well as to electrostatic interaction with sulphate groups of reactants.
Fixation of PHMG on any hydroxilated surface is possible without any chemical processes.
PHMG is well soluble in water, belongs to the 4th group of toxicity, therefore, it is safe in work. PHMG belongs to light preparations manufactured at an affordable price.
Sulphonic derivatives of organic complex-forming reagents were chosen as organic reagents for creating supramolecular assemblies on the surface of inorganic oxides. The structure of the surface layer of aminated CPH (co-precipitated hydroxide) puts restrictions to using organic reactants capable of forming supramolecular assemblies. The main requirement to organic reagents, beside their complex-forming properties, is the necessity of the presence of negatively charged groups in the composition of the organic reactant, through which organic reactants are fixed on the aminated surface.
Organic reactants are fixed due to the electrostatic interaction between the negatively charged groups of the organic reactant and protonated primary amino groups of PHMG not involved in forming hydrogen bonds with hydroxyl groups on the CPH surface.
Thus, in order to create surface supramolecular assemblies with complex-forming properties on the surface of non-organic oxides, 1,8-dioxynaphthalene-3,6-disulfonic acid was chosen as the organic reactant:
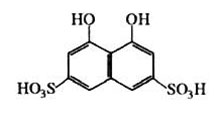
In course of consequent CPH processing with PHMG solution, a supramolecular grid of 3 reactants that are bonded by intermolecular forces is formed on the surface. In this case, the surface of CPH can be represented as a macromolecule with many hydroxyl groups (Fig. 1)
The developed approach to synthesis of sorbents is better than that in case with sorbents with impregnated organic reactants on the surface of solids, and sorbent with chemically fixed groups, which makes it possible to fix a different number of groups with uniform fixation on the surface [26-28].
The granules of magnesium and aluminum CPH obtained during synthesis are a biporous sorbent in the form of agglomerates of crystallites, where ion exchange takes place both on the surface of the crystallites and inside them in the form of diffusion into the interlayer space of crystallites structure.
Granulation by drying at 115-120°C made it possible to obtain a sorbent with high strength that is necessary to retain initial shape of granules during transportation and handling. The geometric shape of sorbents significantly affects the nature of mass transfer processes in the layer, therefore, in our opinion, it is necessary to use the sorbent consisting of 2.5-3 mm granules [8].
Continuous method of obtaining the sorbent allowed withstanding constant pH of precipitation and, adjusting the stoichiometric amounts of initial solutions, to obtain fairly pure precipitates of metals hydroxides without alkaline salts, which allows easier washing-off mother water from adsorbed ions.
The data about efficiency of extracting ions of copper, lead, zinc, nickel and cadmium from single-component solutions are shown in Table 5. Sorbent consumption was 500 mg/l.
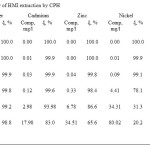 |
Table 5: Efficiency of HMI extraction by CPH Click here to View table |
The analysis of extraction shows that when elements concentration in the solution is between 1 and 10 mg/l, complete (100 %) extraction of metals from treated water is possible.
The result of the experiments in adsorbing heavy metals from single-component solutions with the modified sorbent should be a defined set of active metals, which reflects the ability to absorb some ions stronger than the others (the selectivity phenomenon).
In the row, the sorbent has the highest selectivity to copper ions and the lowest one to lead: Cu2+>Cd2+>Zn2+>Ni2+ >Pb2+
It was found that during interaction of the sorbent with these ions, substitution of magnesium ions with the cations of extracted metals, as well as chemical bonds opening on the surface of the sorbent, formation of aqueous and hydroxocomplexes, and complexes with organic modifiers occur on the surface of the sorbent.
The most common characteristic of the sorbent is its specific surface defined by the total volume and pores size. The specific surface area was determined by low temperature nitrogen adsorption using the chromatographic method of analysis with subsequent data processing according to the Brunauer–Emmett–Teller surface area equation. The obtained results suggest that CPH may be used as an inorganic matrix for the production of the modified sorbent. Adsorption and structural characteristics of the sample at 120°C are shown in Table 6.
Table 6: Adsorption and structural characteristics of samples
|
Sample |
Specific surface, m²/g |
Pore volume, cm³/g |
Effective pore radius, A0 |
|
|
Total |
Small |
|||
|
CPH |
160 |
0.83 |
0.100 |
50-75, 1,150-1,400 |
Effectiveness of using the modified sorbent for wastewater treatment and removing heavy metal ions is confirmed by the tests performed with real wastewater.
The sorption capacity of the sorbents was calculated using the following formula:
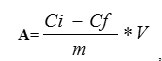
where Ci is the initial concentration of the adsorbate in the solution, mg/l;
Cf is the final concentration of the adsorbate in the solution, mg/l;
m is weighed amount of the sorbent, g; and
V is the volume of model solution, l.
Analyzing obtained data, one can come to the conclusion that sorbents are characterized by high sorption capacity to ions of copper, zinc, and lower capacity – to nickel and lead. Effectiveness of using sorbent for wastewater treatment with removing ions of heavy metals is confirmed by the tests performed with real wastewater. Effectiveness of using the modified sorbent for wastewater treatment and removing heavy metal ions is confirmed by the tests performed with real wastewater. Also, the obtained sorbent should be used for treatment of oil-containing solutions.
Conclusion
A new method of obtaining sorbents based on CPH of aluminum and magnesium has been developed, with consequent modification with polyhexamethylenguanidine and organic complex-forming reactant: 8-hydroxyquinoline-5-sulfonic acid. It has been shown that the ratio of double- and triple-charged cations makes it possible to successfully use this sorbent for quantitative sorption of cations.
Adsorption and structural characteristics of CPH have been defined (specific surface area is 160 m²/g, total pore volume is 0.83 cm³/g), making it possible to offer the synthesized sorbent as a matrix of inorganic ion exchangers.
Optimal conditions for sorption concentration of Cu(II), Cd(II), Pb(Il), Ni(II) and Zn(II) on CPH with modified organic complex-forming reactants have been defined.
It was found that in case of interaction of the sorbent with ions of Cu2+, Zn2+, Cd2+, Pb2+, Ni2+, magnesium ions are replaced with cations of the extracted metals, chemical bonds on the surface of the sorbent are opened, and aqueous and hydro-complexes are formed on the surface of the sorbent, along with complexes with organic modifiers.
The research makes it possible to offer a synthesized sorbent for extracting and concentrating ions of heavy metals from wastewater. Efficiency of the sorbent for wastewater treatment by removing ions of heavy metal is confirmed by the tests performed with real wastewater [24]. Such a trend makes it possible to decrease the anthropogenous pressures on the surface and the underground waterways. The economic effect of introducing the developed technologies can be achieved not only due to commercial effect of introducing new products, but due to environmental and social efficiency as well.
Besides, the complex of research is certainly promising, and will allow to improve the quality of life of living organisms and human health due to significant improvement of consumed water, and to reduce the influence of industrial wastewater on natural and artificial waterways.
References
- A report about the state of environmental management and about environment protection in the Krasnodar territory in. Krasnodar, 2013,. 230
- Bokovikova, T.N., Marchenko, L.A. et alPatent RU #2343120 “Method of wastewater treatment by removing hexacyanoferrates”. Published: 10.01.2009, 2009.
- Cogliastro, A., Domon, G., & Daigle, S., Effects of wastewater sludge and woodchip combinations on soil properties and growth of planted hardwood trees and willows on a restored site. Ecological Engineering, 2001, 16, 471-485.
- Juvonen, R., Martikainen, E., Schuitz, E., Joutti, A., Ahtiainen, J., & Lehtokari, М., A battery of toxicity tests as indicators of decontamination in composting oily waste. Ecotoxicology and Environmental Safety, 2000, 47, 156-166.
- Lau, S., Fang, M., & Wong, J., Effects of composting process and flu ash amendment on phytotoxicity of sewage sludge. Arch. Environ. Contam.Toxicol., 2001, 40, 184-191.
- Kalinin, N.F., Granular sulfide sorbents and their usage. In: Chemistry and technology of inorganic sorbents: Inter-university collection of scientific works 1980; 112-114. Perm.
- Pushkareva, G.I., Sorption extraction of metals from mono – and poly-component solutions using brucite. Physical and Technical Problems in Mining, 1999, 6, 110-113.
- Marchenko, L.A., Marchenko, A.A., Nizhivenko, M.V. et al., Patent RU #2548440 “Method of obtaining modified sorbent for purification of oil-containing water and wastewater”. Published: 20.04.2015. Bull. #11, 2015.
- Volovich, A.I., Sukharev, Y.I., & Kremko, E.G., Synthesis and use of applicated iron hydroxide. In: Chemistry and technology of non-organic sorbents: Abstracts, Ashkhabad 1982, 26-28..
- Volkhin, V.V. Selective non-organic sorbents and their usage. In: Chemistry and technology of non-organic sorbents: Inter-University collection of scientific works (pp. 3). Perm.
- Sukharev, Y.I., & Volovich, A.I., Selenium and tellurium-selective non-organic sorbents. In: Chemistry and technology of non-organic sorbents: Inter-university collection of scientific works Perm 1980; 40-46.
- Donaldson, J.D., & Fuller, M.J., J. Inorg. Nucl. Chem., 1970, 32, 1703.
- Tustanowski, S., J. Chromatog., 1967, 31, 268-270.
- Unnicova, N.A., Sorption of molybdenum (U1), tungsten (VI), and vanadium (V) by hydrated zirconium hydroxide (Candidate’s thesis), LTI, Leningrad, 1977.
- Cvjeticanin, N., & Cvjeticanin, D.M., J. Chromarogr., 1977, 40, 77-85.
- Evtjukhova, O.V., Gorshkova, A.N., Popova, I.A. et al., Research for assessing sorption capacity of natural materials: Theses 17. Proceedings of the Mendeleev Congress on General and Applied Chemistry, Minsk, 1993; 1, pp. 370-371.
- Lvovich, B.I., & Volkhin, V.V., Ion exchange, and ion exchange material: Coll. of articles (pp. 236-240). Leningrad: Nauka, 1970.
- Malykh, T.G., Sharygin, L.M., & Galkin, V.M., The Journal of Applied Chemistry, 1977, 50, 2208-2211.
- Volkhin, V.V., Leontyeva, G.V., & Onorin, S.A., Chemistry and chemical technology. Proceedings of Universities, 1974, 17, 695-698.
- Zosin, A.P., & Priymak, T.I., Treatment of industrial wastewater by removing ions of nickel, cobalt, copper with a sorbent based on magnesium-iron slags from non-ferrous metallurgy. In: Chemistry and technology of non-organic sorbents: Inter-university collection of scientific works Perm 1980; 92.
- Kogai to taisaku., Chemistry abstract journal, J. Environ. Pollut.Confr., 1977, 15, 1099-1106
- Volkhin, V.V. et al., Ion-sieve cation-exchange materials for selective sorption of lithium. Chemistry and technology of non-organic sorbents. In: Chemistry and technology of non-organic sorbents: Inter-University collection of scientific works 1980, 67. Perm.
- Chaly, V.P. , Hydroxides of metals (pp. 141). Kiev: Naukova Dumka, 1972.
- Losev, V.N. et al., Patent RU № 2380152 “Complex-forming sorbent and the method of obtaining it” Published: 2010.
- Kuznetsova, O.V., Using immobilized organic reactants in sorption-optical and chemical methods of testing (Extended abstract from Candidate’s thesis), Moscow, 2000, 23.
- Zaporozhets, O.A., Gaver, O.M., & Sukhan, V.V., Immobilization of analytical reactants on the surface of carrying agents. Successes of Chemistry, 1997; 66(7), 702-712.
- Steed, J.W., & Atwood, J.L., Supramolecular chemistry (Trans., Vol. 1, pp. 480). Moscow: ECC “Akademkniga”, 2007.

This work is licensed under a Creative Commons Attribution 4.0 International License.









