The physico-chemicals analyzes of copolymer Paratone 8900 used as viscosity improvers for SAE 10W-40 mineral oil
Ioana Stanciu
University of Bucharest, Faculty of Chemistry, Department of Physical Chemistry, Bvd. Regina Elisabeta, no. 4-12, 030018 Bucharest, Romania.
Corresponding Athor email: istanciu75@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310315
Article Received on :
Article Accepted on :
Article Published : 15 Sep 2015
Paratone 8900 is used as automotive for as viscosity improvers for multi-grade oils. In this study we established the physico-chemical analyses vized of a copolymer Paratone 8900 used as viscosity improvers for SAE 10W-40 mineral oil was: spectroscopy FTIR, thermo-gravimetric differential thermal analysis (TG-DTA), and the differential scanning calorimetry (DSC). The curve DSC evaluates the glass transition temperature. The thermogrames TG and DTA to evaluates their heat resistance of copolymer Paratone 8900. The kinetic parameters of copolymer Paratone 8900 were determination a method of multilinear regression analysis.
KEYWORDS:DSC; kinetic analysis; multilinear regression; TG-DTA; FTIR
Download this article as:| Copy the following to cite this article: Stanciu I. The physico-chemicals analyzes of copolymer Paratone 8900 used as viscosity improvers for SAE 10W-40 mineral oil. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Stanciu I. The physico-chemicals analyzes of copolymer Paratone 8900 used as viscosity improvers for SAE 10W-40 mineral oil. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10933 |
Introduction
Paratone 8900 is used as automotive for as viscosity improvers for multi-grade oils. The properties chemical and physical of copolymer are: solid, bales, relative density 860-900 kg.m-3, insoluble in water, soluble in alkanes, cicloalkanes, aromatic substances and paraffinic oil.The global and partial solubility parameters and radii of interaction sphere of copolymer Paratone 8900 were : d is 17.6 (MPa)1/2, δd is 17.5 (MPa)1/2, δp is 0.8 (MPa)1/2, δh is 1(MPa)1/2 and R is 4.8 (MPa)1/2 [1-3].
The chemical structure of copolymer Paratone 8900 was determined using spectroscopy FTIR. The differential scanning calorimetry is widely used in the study copolymer Paratone 8900. For the polymer chemist, DSC is a handy tool for studying curing processes, which allows the fine tuning of polymer properties. The cross-linking of polymer molecules that occurs in the occurring process is exothermic, resulting in a positive peak in the DSC curve that usually appears soon after the glass transition [4-6]. The DSC used a determination the glass transition temperature of copolymer Paratone 8900 [6-10].
The object of the present paper is the determination chemical structure using spectroscopy FTIR, glass transition temperature using differential scanning calorimetry (DSC), heat resistance and kinetic parameters using thermo-gravimetric differential thermal analysis (TG-DTA) for a Paratone 8900 copolymer – recommended as viscosity improvers for multi-grade oils. The thermogrames TG and DTA to evaluates their heat resistance of copolymer Paratone 8900. The kinetic parameters of copolymer Paratone 8900 were determination a method of multilinear regression analysis.
Material and Methods
The following copolymer was used as: poly(ethylene-co-propylene) (Exxon Chemical) – trade name Paratone 8900.
Average molecular weight of Paratone 8900 was determined by GPC using a GILSON. The copolymer Paratone 8900 were average molecular weight 1.12 x 105 g · mol-1 and number average molecular weight 4.73 x 104 g · mol-1. Their ratio, which can be taken as a measure of copolymer polydispersity, is 2.36.
FTIR spectra of copolymer measurement using FT-IR GX Perkin Elmer spectrophotometer in the range of 4000 to 500cm-1, with a resolution of 4cm-1.
The curve TG and DTA were determined using DSC DuPont 2000. The experiments were performed in water atmosphere, operated in range 40-600oC and a heating rate 20oC · min-1.
A method of multilinear regression analysis (MLRA) of the kinetic equation was chosen for the simultaneous evaluation of the activation energy, frequency factor and reaction order from a DTA curve. Using a computer simulation program, several DTA curves were obtained, provided with different Gaussian errors, starting from the evaluated kinetic parameters. Since the dependent variable varies within maximum one order of magnitude, constant absolute errors simulate best the naturally occurring spread of experimental data.
Results and Discussion
Figure 1 present spectre FTIR of a copolymer Paratone 8900. The characteristic absorption bands of a copolymer appeared at 2923.14, 1461.08, 1376.62 and 696.06 cm-1. The significant bands, their wavenumbers and the corresponding functional groups are show in Table 1.
Table 1: Significant bands and functional groups of Paratone 8900
|
Wavenumber cm-1 |
Absorbtion Bond |
|
2923.14 |
CH2 asymmetric stretch |
|
1461.08 |
C-CH3 asymmetric stretch |
|
1376.62 |
C-CH3 symmetric stretch |
|
696.06 |
Out of plane CH band |
First, the intensity of the absorption band at 2923.14 cm-1 associated with the CH2 asymmetric stretch [9] had decreased significantly after ages.
The second significant observation about the aged IR spectra is decomposition of the C-CH3 bond with an asymmetric bend at 1461.08 cm-1. The third major observation is in the intensity of the C-CH3 bond with a symmetric bend around wave number 1376.62 cm-1. A similar reduction was also observed for the out-of-plane CH bend absorbtion at wave number 696.06 cm-1.
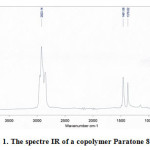 |
Figure 1: The spectre IR of a copolymer Paratone 8900 |
Figure 2 present experimental thermogram of a copolymer Paratone 8900. The experimental thermogram was obtained using a differential scanning calorimeter DSC DuPont 2000. The DSC study was carried out with DSC DuPont 2000 equipment. The experiments were performed in nitrogen atmosphere, operated in the range -80 – 140oC at a heating rate 20oC · min-1 and the sample mass 0.1 mg. The glass transition temperature of copolymer Paratone 8900 is at -54.17oC and at 53.40oC.
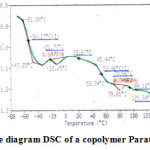 |
Figure 2: The diagram DSC of a copolymer Paratone 8900 Click here to View figure |
The TG and DTA curves in the air atmosphere for a copolymer Paratone 8900 is the present in figure 3.
The figure presents: the modified weight in the temperature range 252-470oC; the temperature initial of a decomposition thermic is 262.5oC; the modified weight with temperature: 262-402.15oC–42.99 %, 402.15-520oC – 54.85%, 520-600oC – 1.465% and rezidue – 0.4249%; the curve present of two maximum, DTG curve we two point of a inflexion, the one of 371.87 and the second 450.96oC and the temperature final on a decomposition thermic is 450oC.
 |
Figure 3: The experimental TG and DTA curves for Paratone 8900 |
The kinetic analysis a thermic degradation of copolymer Paratone 8900 is present paper. The kinetic calculations from the experimental data usually proceed from the basic kinetic equation:
dα/dt = k(T)f(α) (1)
where α is the conversion, t the time and T the absolute temperature. Arrhenius equation is generally used for the dependences of rate constant k(T) on the absolute temperature:
k(T) = Ae-E/RT (2)
where A is the frequency factor, E the activation energy and R the molar gas constant.
The conversion function is dependent on the assumed reaction mechanism. Various from of this function have been published [3-5]. In this work the following from of the conversion function was used:
f(α) = (1- α)n (3)
where n is the reaction order with respect to reactant, describing best our experimental data.
Inserting equations (2) and (3) in equation (1), the kinetic equation in the following form is obtained:
dα/dt = Ae-E/RT(1 – α)n (4)
Under non-isothermal conditions the explicit temporal dependence in equation (4) is eliminated through the transformation:
dα/dt = (A/β)e-E/RTf(α) (5)
where β = dT/dt is the heating rate.
From experimental TGA the weight of temperature can be also determined using for following equation:
dα/dt = Aαn(1-α)me-E/RT (6)
where m is the reaction order.
Starting from experimental DTA curve, the kinetic parameters (E, A, n and m) were evaluated by a multilinear regression method, using the linearized from of equation (6).
Table 2 shows the values of kinetic parameters for the thermic descomposition of poly (ethylene-co-propylene) at 200C min-1 heating rate, obtained by multilinear regression.
Table 2: The kinetic parameters obtained with equation kinetic for the experimental TG and DTA curves of a Paratone 8900
|
Kinetic equation |
Frequency factor, sec-1 |
Activation energy, kJ/mol |
n |
m |
Correlation Coefficient |
|
αn(1 – α)m |
9.891E-01 |
2.188E+01 |
0.52 |
0.98 |
0.9426 |
|
αn |
1.593E-15 |
-1.746E+02 |
2.37 |
– |
0.9584 |
The negative value obtained for activation energy of the thermic decomposition with equation (4) has not a physical sense, which means that the kinetic function is not adequate for is description.
The value that is higger than one reaction order can be explained through average molecular polydispersity, can guide to such stoechiometry.
The mechanism thermic degradation of a copolymer poly (ethylene-co-propylene) is:
- In the temperature range 0-270oC of copolymer is stable thermic,
- In the temperature range 262 and 400oC of degradation thermic with elimination propylene.
- In the 400 and 520oC the contiuue degradation with elimination ethylene.
The composition copolymer of Paratone 8900 is: 54.85 % propylene and 42.99 % ethylene and 1.465 % other aliphatic compounds.
The spectre IR of a copolymer Paratone 8900: CH3, CH2 and CH aliphatic band. The copolymer was the glass transition temperatures -54.2oC and at 53.40oC. The temperature initial of a thermic decomposition is 262 0C and temperature final 520oC. The composition copolymer of Paratone 8900 is: 54.85 % propylene and 42.99 % ethylene and 1.465 % other aliphatic compounds. The copolymer Paratone 8900 as having a bit lower glass transition temperature and higher heat resistance.
References
- Lashkhi, V. L.; Leimeter, S. T.; Shor, G. I.; Fal’kovich, M. I. Chemistry and technology of fuels and oils 2001, 37(5), 372-376.
- Darensbourg, D. J.; Frantz, E. B. Inorganic chemistry 2007, 46(15), 5967-5978.
- Ghari, H. S.; Shakouri, Z. Science and Technology 2011, 24(3), 215-230.
- Karagiaridi, O.; Lalonde, M. B.; Bury, W.; Sarjeant, A. A.; Farha, O. K.; Hupp, J. T. Journal of the American Chemical Society 2012, 134(45), 18790-18796.
- Hao, L.; Mansour, M. A.; Lachicotte, R. J.; Gysling, H. J.; Eisenberg, R. Inorganic chemistry 2000, 39(24), 5520-5529.
- Alawisi, H.; Li, B.; He, Y.; Arman, H. D.; Asiri, A. M.; Wang, H.; Chen, B. Crystal Growth & Design 2014, 14(5), 2522-2526.
- Tang, M.; Li, D.; Mallik, U. P.; Zhang, Y. Z.; Clérac, R.; Yee, G. T.; Holmes, S. M. Inorganic chemistry 2011, 50(11), 5153-5164.
- Mowla, D.; Naderi, A. Chemical Engineering Science 2006, 61(5), 1549-1554.
- Burger, E. D.; Munk, W. R.; Wahl, H. A. Journal of Petroleum Technology 1982, 34(02), 377-386.
- Machado, A. L.; Lucas, E. F.; González, G. Journal of Petroleum Science and Engineering 2001,32(2), 159-165.

This work is licensed under a Creative Commons Attribution 4.0 International License.









