Synthesis, Characterization and antimicrobial evaluation of New Chalcone Derivatives From3- benzyloxy-4-methoxybenzaldehyde
L. Benmekhbi1,2, S. Mosbah1, A. Khelifa Baghdouch1, L. Bencharif1
1Laboratory of molecular chemistry University of Frères Mentouri Constantine1 Algeria.
2Department of Chemistry University of Mohamed boudiaf M’sila Algeria.
Correspondence Author Email: mekhbi@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/310317
Article Received on :
Article Accepted on :
Article Published : 07 Aug 2015
A series of chalcone derivatives (2a–i) were prepared via the reaction of 3-benzyloxy-4-methoxybenzaldehyde with the appropriately acetophenon derivatives. The structures of all the newchalcone derivatives (2a–i) synthesized in this study were established on the basis of 1H NMR and 13C NMR spectral data, and elemental analyses The antibacterial activitie of the synthesized compounds (2a- i) was carried out by well diffusion and MIC method.
KEYWORDS:chalcone; X-ray analysis; Claisen–Schmidt Condensation; antibacterial activitie
Download this article as:| Copy the following to cite this article: Benmekhbi L, Mosbah S, Baghdouch A. K, Bencharif L. Synthesis, Characterization and antimicrobial evaluation of New Chalcone Derivatives From3- benzyloxy-4-methoxybenzaldehyde. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Benmekhbi L, Mosbah S, Baghdouch A. K, Bencharif L. Synthesis, Characterization and antimicrobial evaluation of New Chalcone Derivatives From3- benzyloxy-4-methoxybenzaldehyde. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10190 |
Introduction
Chalcones are products of condensation of simple or substituted aromatic with simple or substituted acetophenones in presence of alkali. Chalcone constitute an impartment group of natural products and some of them possess a wide range of biological activities such as anticancer1 antitubercular2,antiviral3, also they are used as anti-malarial4, anti protozoal5, anti-inflammatory6,immunomodulatory7-8, nitric oxid inhibition9,tyronase inhibition 10, cytotoxic11, antimicrobial12, Geiger and Conn13 during their chemical studies on the structure of clavicin found that a structural feature which was responsible for antibacterial activity was α, β unsaturated keto functional group. These molecules are also used as starting materials in the synthesis of UV absorption filters in polymers, photo refractive polymers, photosensitizers in Bcolor films, sweeteners in food technology, and in holographic recording technology. A natural medicine genus Angelica is known to contain large number of naturally occurring chalcones14.Chalcone derivatives are recognized for NLO properties and have good crystallization ability15Structure of few related chalcones viz., (2E)-3- (biphenyl-4-yl)-1-(4-methoxyphenyl)prop- 2-en-1-one (Fischer et al., 2007)16, (E)-3-(2,6-Dichlorophenyl)-1-(4-
Pharmacological properties of chalcones are due to the presence of both α,β, unsaturation18 and an aromatic ring. Constant interest in chalcones has resulted in syntheses of new derivatives using both classical19-20 and combinatorial techniques21.
In this study, a series of new chalcone-like compound (2a–i) were synthesized by the reaction of 3-benzyloxy-4-methoxybenzaldehyde with the appropriately acetophenon derivatives.The structures of all the chalcone derivatives (2a–i) synthesized in this study were established on the basis of 1H NMR and 13C NMR spectral data, and elemental analyses.The structure of compound 2i was further confirmed by X-ray analysis of single crystal.
Results and Discussion
Melting points of the compound were measured using an Electrothermal 9100 apparatus. IR spectrums (KBr or liquid) were taken by a Jasco FT=IR-430 IR spectrophotometer.1H and 13C NMR spectra were recorded using a Brucker Avance III instrument using tetramethylsilane (TMS, d 0.00) for 1H NMR and DMSO for 13C NMR spectroscopy as internal reference standards; J values were given in hertz. The multiplicities of the signals in the 1H methoxyphenyl)prop-2-en-1-one (Benmekhbi et al., 2009)17 .
NMR spectra are abbreviated by s (singlet), d (doublet), t (triplet), q (quarted), m (multiplet),
Reagent
3-benzyloxy-4-methoxybenzaldehyde and appropriately acetophenon derivatives were commercial products with the highest reagent grade.
Chemistry
To a mixture of 3-(benzyloxy)-4-methoxybenzaldehyde (2g, 0.008 mol) and appropriately acetophenon derivatives (0.008 mol) in éthanol 20 ml in the presence of a catalytic amount of sodium hydroxide solution (5 ml) was added slowly with stirring (6 h), neutralized with HCl solution (10%) the contents of the flask were poured into ice cold water (500 ml) and left to stand for 5 h,the organic layer was dried over anhydrous Na2SO4,(Scheme 1)The resulting crude solid was filtered and purified by recrystallization in ethanol. The structure of the compound 9(2i) was further confirmed by X-ray analysis of single crystal. Crystal suitable for x-ray analysis was grown by slow evaporation of a mixture acetone/ ethanol solution at room temperature. The crystal used for data Collection was of the dimension) 0.51 x 0.31×0.15 mm
 |
Table 1: The new chalcone derivatives Click here to View table |
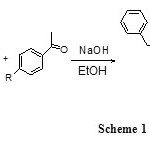 |
Scheme 1 Click here to View scheme |
X- ray analysis
The compound, 2i( C23H19ClO3 ), exists in an E conformation with respect to the C=C bond. The central benzene ring forms a dihedral angle of 88.96 (2)° with the chlorobenzene ring and a dihedral angle of 22.53 (2)° with the terminal benzene ring. No significant intermolecular interactions are observed (fig1, fig2).
Refinement
All H atoms were localized in Fourier maps but introduced in calculated positions and treated as riding on their parent C atoms with C—H = 0.95Å to 0.99Å and Uiso(H) = 1.2 or 1.5Ueq(C).
Crystallographic data and details of the data collection and structure solution and refinements are listed in Table 2
Table 2: Crystallographic Data Collection and Structure Refinement Parameters of2i
| Crystal data | Z = 2F(000) = 396
Dx= 1.324 Mg m−3 Mo Kα radiation, λ = 0.71073 Å Cell parameters from 2479 reflections θ = 2.7–27.4° μ = 0.22 mm−1 T = 150 K Prism, colourless 0.51 × 0.32 × 0.15 mm
|
| C23H19ClO3Mr= 378.83Triclinic, P1
Hall symbol: -P 1 a = 7.9049 (4) Å b = 10.9621 (5) Å c = 11.8899 (5) Å α = 102.956 (2)° β = 105.739 (2)° γ = 96.418 (2)° V = 949.93 (8) Å3
|
|
| Data collection | 8538 measured reflections4333 independent reflections
3336 reflections with I > 2σ(I) Rint= 0.027 θmax = 27.5°, θmin = 3.0° h = −10→10 k = −12→14 l = −15→12
|
| APEXII, Bruker-AXSdiffractometerGraphite monochromator
CCD rotation images, thin slices scans Absorption correction: multi-scan [Sheldrick, G.M. (2002). SADABS Bruker AXS Inc., Madison, Wisconsin, USA] Tmin= 0.884, Tmax= 0.967 |
|
| Refinement | Secondary atom site location: difference Fourier map Hydrogen site location: inferred from neighbouring sites H-atom parameters constrainedw = 1/[σ2(Fo
2) + (0.0425P)2 + 0.2095P] where P = (Fo 2 + 2Fc 2)/3 (Δ/σ)max = 0.002 Δρmax = 0.25 e Å−3 Δρmin= −0.26 e Å |
| Refinement on F2Least-squares matrix: fullR[F2 > 2σ(F2)] = 0.043
wR(F2) = 0.107 S = 1.04 4333 reflections 245 parameters 0 restraints Primary atom site location: structure-invariant directmethods
|
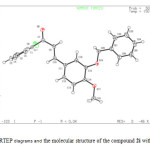 |
Figure 1: The ORTEP diagrams and the molecular structure of the compound 2i with atom labels. |
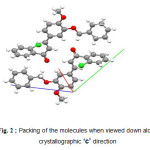 |
Figure2: Packing of the molecules when viewed down along crystallographic ‘c’ direction |
Data
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-phenylprop-2-en-1-one (2a)
Viscousoilbp201-210°C1HNMR(300MHZ, DMSO)α=7,90(d, J=15,5, 1H, CH=), 7,45-7,84(m, 5H,ArH, 2H,CH=CH), 7,56(d,J=15,5, 1H, =CH), 7,19(S, 5H,ArH), 6,62-6,74(m, 3H, Ar), 5,20(S,2H, CH2-O), 3,81(S,3H,CH3-O)13C NMR(300MHZ, DMSO)α=189,71, 149,7, 149, 145,2, 141, 137,2, 134,6, 129,9, 129,2 (2C),127,7, 127,2 (2C), 121,2, 119,7, 115,2, 111, 72,2, 56,2.IR (liquid):31353032, 3009,2811, 1683, 1656, 1525,1457, 1367, 1056, 806,775, 733Anal.calcd. for C23H20O3C, 80.21; H, 5.85; O, 13.94. Found C, 80.05; H, 5.90; O, 14.01.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(4-chlorophenyl)prop-2-en-1-one (2b)
Yellowish crystals, mp145–150°C.1H NMR(300MHZ, DMSO)α=8,20(d, J=8,5, 2H), 7,96(d, J=8,5, 2H), 7,84(d, j=15,5, 1H), 7,74(d,J=15,5, 1H), 7,73(d, J=1,2, 1H), 7,68(d, J=8,5, 2H), 7,6(d, J=8,4, 1H), 7,52(d, J=8,4, 2H), 7,35-7,45(m, 2H), 7,08(d, J=8,4, 1H), 5,20(s, 2H), 3,81(s,3H) 13C NMR(300MHZ, DMSO)α=189,71, 149,7, 149, 145,2,141,2, 140,1, 136, 131,3(2C), 129,4(2C),129(2C), 128,5, 127,7 ,127,2(2C), 121,4, 119,7, 115,2, 111,6, 71,2, 56,2. IR (KBr):3125,3112, 3019, 2819, 1673, 1662, 1535,1551, 1467, 1368, 1150, 816, 765, 723 Anal. calcd. for C23H19ClO3C, 72.92; H, 5.06; Cl, 9.36; O, 12.67. FoundC, 72.90; H, 5.08; Cl, 9.26; O, 12.77.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(4-methoxyphenyl)prop-2-en-1-one (2c)
Yellowish crystals, mp135–140°C.1H NMR(300MHZ, DMSO)α=8,18(d, J=8,5, 2H), 7,94(d, J=8,5, 2H), 7,80(d, j=15,5, 1H), 7,77(d, J=15,5, 1H), 7,75(d, J=1,2, 1H), 7,62(d, J=8,5, 2H), 7,59(d, J=8,4, 1H), 7,52(d, J=8,4, 2H), 7,30-7,41(m, 2H), 7,01(d, J=8,4, 1H), 5,28(s, 2H), 3,77 (s,6H) 13C NMR(300MHZ, DMSO)α=189,71, 149,5, 148, 144,2,142,2, 141,1, 135, 130,3(2C), 128,4(2C),128(2C), 127,7, 127,3 ,126(2C), 120,4, 119, 114,2, 112,6, 70,2, 56,2, 54,2. IR (KBr): 31123100, 3049, 2779, 1713, 1622, 1515, 1460, 1328, 1159,1032 , 806, 735, 703 Anal. calcd. For C24H22O4 C, 76.99; H, 5.92; O, 17.09 Found C, 76.89; H, 5.90; O, 17.21.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-p-tolylprop-2-en-1-one (2d)
Yellowish crystals, mp143–150°C .1H NMR(300MHZ, DMSO)α=8,12(d, J=8,5, 2H), 7,90(d, J=8,5, 2H), 7,80(d, J=15,5, 1H), 7,76(d,J=15,5, 1H), 7,73(d, J=1,2, 1H), 7,65(d, J=8,5, 2H), 7,61(d, J=8,4, 1H), 7,502(d, J=8,4, 2H), 7,33-7,41(m, 2H), 7,12(d, J=8,4, 1H), 5,13(s, 2H), 3,81(s,3H), 2,35(s,3H), 13C NMR(300MHZ, DMSO)α=189,61, 149,61, 148, 145,22,142,2, 140,15, 136, 131,3(2C), 129,11(2C), 129(2C), 128,51, 127,77 ,127,21(2C), 121,11, 119,17, 115,12, 110,6, 71,2, 56,2, 24,55. IR (KBr): 3120, 3102, 3009, 2719, 1603, 1592, 1555, 1487, 1338, 1110,1054, 819, 735, 703 Anal. calcd. For C24H22O3C, 80.42; H, 6.19; O, 13.39. Found C, 80.32; H, 6.24; O, 13.44.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one (2e)
White solid146–150°C 1H NMR(300MHZ, DMSO)α=7,96(d, J=8,5, 2H), 7,90(d, J=8,5, 2H), 7,81(d, j=15,5, 1H), 7,74(d, J=15,5, 1H), 7,72(d, J=1,2, 1H), 7,68(d, J=8,5, 2H), 7,60(d, J=8,4, 1H), 7,52(d, J=8,4, 2H), 7,31-7,40(m, 2H), 7,18(d, J=8,4, 1H), 5,10(s, 2H), 5,02(s,1H), 3,35(s,3H), 13C NMR(300MHZ, DMSO)α=189,61, 149,61, 148, 145,22, 142,2, 140,15, 136, 131,3(2C), 129,11(2C), 129(2C), 128,57, 127,54 ,127,10(2C), 122,11, 118,14, 115,12, 111,6, 71,2, 56,2. IR (KBr): 3572,31053130, 3033, 2111, 1653, 1612, 1515, 1411, 1342, 1122, 832, 722, 711 Anal. calcd. ForC23H20O4 C, 76.65; H, 5.59; O, 17.76.Found C, 76.35; H, 5.79; O, 17.86.
(E)-1-(4-aminophenyl)-3-(3-(benzyloxy)-4-methoxyphenyl)prop-2-en-1-one (2f)
White solid, mp152–158°C1H NMR(300MHZ, DMSO)α=8,01(d, J=8,5, 2H), 7,92(d, J=8,5, 2H), 7,72(d, J=15,5, 1H), 7,70(d, J=1,2, 1H), 7,64(d, J=8,5, 2H), 7,60(d, J=8,4, 1H), 7,50(d, J=8,4, 2H), 7,33-7,45(m, 2H), 7,20(d, J=8,4, 1H), 5,11(s, 2H), 4,02(s,2H), 3,35(s,3H), 13C NMR(300MHZ, DMSO)α=187,61, 147,61, 146,11, 144,22, 142,21, 138,15, 136,12, 131,30(2C), 129,16(2C), 128,60(2C), 128,17, 127,54(2C),121,29, 122,11, 119,14, 115,12, 110,6, 71,2, 56,2. IR (KBr): 31013052, 3019, 2519, 1673,1661, 1642, 1525, 1437, 1343, 1152, 810, 715, 702 Anal. calcd. For C23H21NO3.C, 76.86; H, 5.89; N, 3.90; O, 13.35.FoundC, 76.76; H, 5.99; N, 3.75; O, 13.50.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(4-bromophenyl)prop-2-en-1-one (2g)
Yellowish crystals, mp 155–157°C .1H NMR(300MHZ, DMSO)α=8,16(d, J=8,5, 2H), 7,92(d, J=8,5, 2H), 7,84(d, J=15,5, 1H), 7,72(d, J=15,5, 1H), 7,71(d, J=1,2, 1H), 7,66(d, J=8,5, 2H), 7,61(d, J=8,4, 1H), 7,50(d, J=8,4, 2H), 7,35-7,45(m, 2H), 7,08(d, J=8,4, 1H), 5,20(s, 2H), 3,81(s,3H) 13C NMR(300MHZ, DMSO)α=189,71, 149,7, 149, 145,2,141,2, 140,1, 136, 131,3(2C), 129,4(2C),129(2C), 128,5, 127,7 ,127,10(2C), 121,44, 119,7, 115,2, 110,6, 70,9, 55,90. IR (KBr): 31203122, 3033, 2822, 1677, 1632, 1513, 1451, 1360, 1159, 801, 745, 714,665 Anal. calcd. For.C23H19BrO3 C, 65.26; H, 4.52; Br, 18.88; O, 11.34.Found C, 65.06; H, 4.62; Br, 18.90; O, 11.42.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(4-nitrophenyl)prop-2-en-1-one (2h)
White solid, mp152–156°C1H NMR(300MHZ, DMSO)α=8,22(d, J=8,5, 2H), 7,97(d, J=8,5, 2H), 7,88(d, J=15,5, 1H), 7,74(d,J=15,5, 1H), 7,72(d, J=1,2, 1H), 7,69(d, J=8,5, 2H), 7,64(d, J=8,4, 1H), 7,52(d, J=8,4, 2H), 7,34-7,46(m, 2H), 7,12(d, J=8,4, 1H), 5,22(s, 2H), 3,81(s,3H) 13C NMR(300MHZ, DMSO)α=190,11, 148,7, 149, 145,71,142,2, 140,16, 136, 131,33(2C), 129,48(2C),129(2C), 128,59, 127,77 ,127,22, 121,44, 119,7, 111,24, 74,66, 55,96. IR (KBr): 3111,3101, 2919, 2713, 1643, 1645, 1563, 1412, 1368, 1334,1110, 801, 712, 701.Anal.calcd. For.C23H19NO5C, 70.94; H, 4.92; N, 3.60; O, 20.54 Found. C, 70.54; H, 5.02; N, 3.70; O, 20.74.
(E)-3-(3-(benzyloxy)-4-methoxyphenyl)-1-(2-chlorophenyl)prop-2-en-1-one (2i)
Yellowish crystals, mp160–165_C. 1H NMR (300MHZ, DMSO)α=7,80(d,J=15,5, 1H), 7.46-7.75 (m, 4H), 7.56(d, J=15,5, 1H), 7.19(m, 5H),6.61-6.75(m, 3H), 5.20(s, 2H), 3,81(s,3H) 13C NMR(300MHZ, DMSO)α=190,11,149,7, 149, 145,11, 141,12, 137,3, 136,4, 134,2, 131,3, 129,4, 129(2C),128,5, 127,7, 127,4, 127,2(2C),120, 115, 111,5, 71,2, 56,4 IR (KBr): 3111,3101, 2901, 2821, 1693, 1652, 1531,1512, 1411, 1345, 1144, 801, 715, 701 Anal. calcd. for C23H19ClO3 C, 72.72; H, 5.16; Cl, 9.41; O, 12.72 FoundC, 72.90; H, 5.08; Cl, 9.38; O, 12.69.
Antibacterial Bioassay
Derivitives 2 (a-i) were tested for in vitro anti-microbial activity against five different bacterial species (Gram negative and Gram positiv) namely:
Staphylococcus aures ATCC, Klebsiela pneumonia ATCC, Escherichia coli ATCC, Pseudomonas aeruginosa ATCC, Proteus mirabilis ATCC using: the diffusion method and the minimum inhibitory concentration (MIC).
The diffusion méthode (methode of the disk):
Each disk contain 100mg of the test compound for this method Muller Hinton agar was melted at 100C° and after cooling to 56 C° was poured into Petri plates of 9cm diameter in quantities of 18 ml, left on the flat surface to solidify and the surface of the medium was dried at 37C°, then the culture of each bacteria and yeast strain after being kept in Mueller –Hinton broth to 10-5 cfu ml-1 were pipetted into the Mueller-Hinton agar plate prepared as described above, the surface of the medium was allowed to dry . The 10 mg ml-1 in DMSO compound impergnted discs were applied to the surface of incubated plates. The Petri plates were placed in an incubator at 37C° after 18h of incubation the Petri plates were examined and it was found that all the test compounds exhibited different degrees of antibacterial activity or inhibitory action (table3).
The minimum inhibitory concentration (MIC)
The MIC of these compounds was determined by the micro-broth dilution technique using Muller-Hinton Broth. Serial tow –fold dilution ranged from 2500 to 2.4 μg-1 for compounds.
The inoculums was prepared in broth which had been kept overnight at 37C° and which had been diluted with Muller –Hinton Broth to give a final concentration of 10-5 mg.ml-1 in the test tray. The trays were covred and placed in plastique bags to prevent drying after incubation at 37C° for 18-24h. The MIC was defined as the lowest concentration of compound giving complet inhibition of visible growth (table4).
Table 3: Antibacterial test of the synthesized compounds by disc diffusion method against tested strains
|
Compound No |
Inhibition zone (diameter) mm of synthesized compound
|
||||
| Escherichia Coli | PseudomonasAeruginosa |
Klebsiella Pneumoniae |
ProteusMirabilis | Staphylococcus aureus | |
|
2a |
12 |
14 | 14 | 12 |
12 |
|
2b |
14 |
20 | 16 | – |
10 |
|
2c |
20 |
18 | 20 | 14 |
18 |
|
2d |
18 |
16 | 22 | 10 |
12 |
|
2e |
20 |
16 | 14 | 12 |
18 |
|
2f |
12 |
10 | 14 | 18 |
20 |
|
2g |
10 |
12 | – | 12 |
10 |
|
2h |
18 |
– | 16 | – |
16 |
|
2i |
20 |
14 | 18 | 18 |
16 |
Table 4: Minimal inhibitory concentration (MIC) in μg.ml-1 of synthesized compounds against tested strains
|
Compound No |
Minimal inhibitory concentration (MIC) in μg.ml-1 |
||||
| Escherichia Coli | PseudomonasAeruginosa | KlebsiellaPneumoniae | ProteusMirabilis | Staphylococcus aureus | |
|
2a |
17 |
14 |
14 |
17 |
12 |
|
2b |
14 |
17 |
16 |
– |
10 |
|
2c |
19 |
18 |
19 |
14 |
16 |
|
2d |
17 |
16 |
21 |
15 |
15 |
|
2e |
17 |
16 |
14 |
12 |
15 |
|
2f |
12 |
10 |
14 |
18 |
17 |
|
2g |
11 |
10 |
– |
11 |
11 |
|
2h |
18 |
– |
16 |
– |
16 |
|
2i |
20 |
18 |
19 |
18 |
17 |
Antibacterial Evaluation
The antibacterial evaluation data for compounds (2a-i) is presented in Table-3 and Table-4. The zone of inhibition was measured in mm, Minimal inhibitory activity was observed for 2500 μg/mL to 2.4 μg-1 and compounds showed their effect in a dose dependant manner. The antibacterial activity of the different compound is moderate, good and excellent
From table-3, it is observed that compound 2i with 2-Chloro substitution, compound 2c with 4-methoxy substitution and 2d with 4-Methyl, exhibited excellent antibacterial activity against the gram positive and gram negative bacterial species.All the other tested compounds exhibited different degrees of antibacterial activities, and the inhibition actions were between 10 to 18mm.The moderate antibacterial activity was recorded for the compound 2g with all bacterial tested species.
Conclusion
Nine novel chalcone derivatives (2a–i) were synthesized by the Claisen-Schmidt condensation the structural confirmation of these derivatives (2a-i) was accomplished by spectroscopic techniques, including 1H NMR, 13C NMR, IR and elemental analyses.
The antibacterial activitie of the synthesized compound (2a- i) were carried
out by well diffusion and MIC method. The obtained results proved that the synthesized chalcones analogues have diffrent antimicrobial effects against all the bacterial specis, and some product (2c, 2d, 2i) exhibited excellent antibacterial activity against the gram positive and gram negative bacterial species.
Acknowledgment
The authors wish to thank University of rennes1 for providing research facility, Dr Thierry Roisnel, Centre de Diffractome´ trie X (CDIFX) de Rennes1, France, for the data-collection facilities. University of M’sila and Constantine1 for financial support for the accomplishment of this work.
References
- Konieczny M. T.,Horowska, B.; Kunikowski. A.,Konopa. J., Wierzba. K., Yamada. Y., Asao. T., Synthesis of polyhydroxylated derivatives of phenyl vinyl sulfone as structural analogs of chalcones. Synthsis2001, 9, 1363–1367.
- Shivakumar.P.M .Geetha, S. M ,Mukesh, D.,Chemical and Pharmaceutical Bulleti2005, 55: 44-49.
- Churkin D., PanfilovaL. V.,Boreko, E.I. Pharm. Chem. 1982. 16: 103-105.
- Liu M., Wilairat P.,Cropft S. L., Tan A. L. C. & Go M. L. Bioorg. Med. Chem. 2003.11, 2729–2733.
- Li R., Kenyon G. L., Cohen F. E., Chen X., Gong B., Dominguez J. N., Davidson E., Nuzum E.O., Rosenthal P. J., & McKerrow J. H. J.,Med. Chem. 1995. 38, 5031–5033.
- Hsieh H. K., Lee T. H. , Wang J. P., Wang J. J.,& Lin C. N. Pharm. Res. 1998. 15, 39–46.
- Barford L., Kemp, K. , Hansen M. &Kharazmi A., Int. Immunopharm. 2002. 2, 545–550.
- Rojas J., Paya M., Dominguez J. N. &Ferrandiz M. L., Bioorg. Med. Chem. Lett. 2002.12, 1951–1953.
- Nerya O., Musa R., Khatib S., Tamir S., & Jacob O., Phytochemistry, 2006.65, 1389–1393.
- Yang Y. , Xia P.,Bastow K. F., Nakanishi Y. & Lee K. H.,Bioorg. Med. Chem. Lett. 2000. 10, 699–701.
- Prasad Y., Praveen K. P., Ravi Kumar P., Eur. J. Chem. 2008.5: 144-148.
- Walton B., Geiger & Jean E.C.,J. Am. Chem.Soc. 1945. 67 : 112.
- Sarker S. D.,&Nahar L.,Curr. Med. Chem. 2004.11, 1479–1500.
- Goto Y., Hayashi A., Kimura Y. &Nakayama M. J., Cryst. Growth, 1991. 108, 688–698.Karat, P. P. &SarojiniB. K. J., Cryst. Growth, 2002. 242, 209–214,Sarojini, B. K.,Narayana B., Ashalatha B. V., Indira J. & Lobo, K. G. J. Cryst. Growth, 2006.295, 54–59.
- Fischer A.; Yathirajan H. S.,Ashalatha B. V.,Narayana B. &Sarojini B. K. Acta Cryst. E63,2007., 1349–o1350.
- Benmekhbi L., Belhouas R., Bouacida S.; Mosbah, S., & Bencharif L., Acta Cryst. E65,2009, o1472-o1473.
- Furusawa M., Tanaka T., Ito T., Nishiwaka A.,Yamazaki N.,Nakaya K. I., Matsuura N., Tsuchiya H., Nagayama M., Iinuma M., J. Health Sci. 2005, 51, 376–378.
- Powers D. G.,Casebier D. S., Fokas D., Ryan W. J., Troth J. R., Coffen D. L., Tetrahedron. 1998, 54, 4085–4096.
- Wattanasin S., Murphy W. S., Synthesis. 1980, 647–650.
- Maha M.A. Khalifa., Oriental Journal of Chemistry, 2008, 24, 825-830.
- Naresh K, Geetha R, Arthikyan J.K Rajasekhar C., Oriental Journal of Chemistry 2014, 30, 1083-1098

This work is licensed under a Creative Commons Attribution 4.0 International License.









