Nanocomposite hydrogels based on water soluble polymer and montmorillonite-Na+
Fatiha Reguieg, Nabahat Sahli, Mohammed Belbachir
Chemistry laboratory of polymer, Faculty of Sciences Exactes and Appliquees , Oran University. Bp 1524 El Mnouar 31000 Oran.
Coresspondence Author Emails: fatiha_reguieg@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/310343
Article Received on :
Article Accepted on :
Article Published : 25 Jul 2015
A series of composites hydrogels based on Poly (1,3-dioxolane) (PDXL),water soluble polymer, were synthesized directly in water by free-radical homopolymerization of a,w-methacryloyloxy PDXL macromonomers using hydrophilic sodium Montmorillonite clay: Maghnite-Na+ (Mag-Na+) and potassium persulfate as an initiator. These materials were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (ATG) and their equilibrium swelling behavior in water and were compared with those of pure hydrogels prepared without Mag-Na+. X-ray diffraction and Infrared spectroscopy confirmed insertion of clay into polymer. The thermal decomposition temperature of the hydrogels based on maghnite-Na+ was found to be higher than of pure hydrogels. At the same time, the influence of the macromonomer precursor molar mass value, its concentration and the quantities of Mag-Na+, on the values of the volume degree of equilibrium swelling were studied. The results showed that the volume degree of equilibrium swelling was investigated as a function of the clay content. However, whether the concentration of macromonomer precursor increased, the volume or weight degree of equilibrium swelling of hydrogels all decreased. The addition of Mag-Na+ particles changed the crosslinking density of hydrogels.
KEYWORDS:poly(1,3-dioxolane); macromonomers; hydrogel nanocomposite; maghnite-H+; maghnite-Na+
Download this article as:| Copy the following to cite this article: Reguieg F, Sahli N, Belbachir M. Nanocomposite hydrogels based on water soluble polymer and montmorillonite-Na+. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Reguieg F, Sahli N, Belbachir M. Nanocomposite hydrogels based on water soluble polymer and montmorillonite-Na+. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10040 |
Introduction
Hydrogels are three dimensional lattices containing hydrophilic polymer chains connected together via covalent or reversible crosslinking points (1-4). They can swell, absorb a large amount of water in a short time and retain water under pressure. When hydrogels are placed in aqueous medium, they therefore swell to several times their initial volume without either dissolving or changing their shape (5). Owing to their advantageous properties, hydrogels are widely applied in medicine (controlled drug release, wound treatment, skin expansion, contact lenses, biosensors)(6-9), as well as in other many fields, such as hygienic products(10), in agriculture(11) and horticulture(12), sealing(13), drag delivery systems (14,15) and local dewarting(16) and stimulus responsive materials (17)
Different reactions used to prepare permanent hydrogels are irradiation (18) or end linking procedures (19). Their synthesis by free radical polymerization of bifunctional macromonomers (20) is easy to perform, applicable directly in water and nevertheless yields hydrogels with a controlled structure (21-22).
In recent years, the preparation of organic–inorganic hydrogel composites (23, 24) has attracted great attention (25, 26, 27) because of their relatively low production cost, high water absorbency and their considerable range of applications in agriculture and horticulture. Compared with conventional organic hydrogels, organic-inorganic nanocomposites hydrogels generally exhibit markedly improved mechanical properties (28), salt resistance (29) and low cost (30)
By introducing the clay on the preparation of hydrogels, composites hydrogels were obtained with excellent mechanical performance (16). Different composites hydrogels based on various clay, such as poly(sodiumacrylate)/sepiolite(31), poly(acrylicacid)/montmorillonite(32), poly(Nisopropylacrylamide)/hectorite(33), poly(Nisopropylacrylamide)/laponite(33), poly(acrylic acid/mica) (34) and polyacrylamide/kaolinite (35) were prepared. Because of their hydrophilic nature, clays are suitable for use in water absorbents as additives (36).
The type of silicate used to crosslink the precursor affects the swelling properties and thermal stability of the hydrogel. Therefore, the crosslinker silicate chosen should be based on the application of the hydrogel.
For example, a montmorillonite crosslinker should be chosen for application in which a fast swelling rate is needed. But a mica crosslinker should be chosen if the hydrogel needs to swell-deswell multiple times (37).
Montmorillonite (MMT) is main natural mineral clay widely used to prepare composite hydrogel, due to their good water absorption, extensive swelling in water and cationic exchange capacity (38). Montmorillonite is a sedimentary rock consisting to a large proportion of clay minerals with a typical 2:1 layered structure (smectites). Montmorillonite has sandwich type structure with one octahedral aluminium sheet and two tetrahedral silicium sheets.
The used clay in this work is sodium Montmorillonite (MMT-Na+). Sodium montmorillonite (MMT-Na+) is a naturally occurring 2:1phyllo-silicate, capable of forming stable suspension in water. This hydrophilic character of MMT-Na+ also promotes dispersion of these inorganic crystalline layers in water soluble polymers such as poly(vinyl alcohol) and poly(ethylene oxide).
In the present work, we used Algerian Montmorillonite clay called Maghnite (39), a new non-toxic initiator, was used as catalyst for cationic polymerization of a number of vinylic and heterocyclic monomers (39–42). The water soluble polymer used is Poly(1,3-dioxolane).
Two cases will be considered: the preparation of pure PDXL hydrogels and that of composite hydrogels based on PDXL with hydrophilic sodium Montmorillonite clay: sodium exchanged montmorillonite clay called maghnite-Na+ (Mag-Na+) in aqueous solution, using potassium persulfate as an initiator. For these synthesis, we prepared in the first step the bifunctional poly (1,3-dioxolane) (PDXL) precursor : a,w-methacryloyloxy PDXL macromonomers by cationic ring opening polymerization of 1,3-dioxolane in the presence of methacrylic anhydride and proton exchanged montmorillonite clay called maghnite-H+ (Mag-H+) as catalyst in the bulk at 20°C.
The second part is concerned with the structural properties of PDXL hydrogels and hydrogel nanocomposites and the influence of the macromonomer molar mass value, its concentration and the quantities of Mag-Na+, on the values of the volume degree of equilibrium swelling.
Experimental
Reagent
1,3-Dioxolane (DXL) (96%), methacrylic anhydride (94%), tetrahydrofurane (THF) (99%), dichloromethane and ethanol ((98%), potassium persulfate (K2S2O8), were purchased from Aldrich and used as received.
Preparation of maghnite-H+
Pristine Maghnite is activated with a sulfuric acid solution to give a Maghnite exchanged with protons, called Mag-H+. The preparation of the Mag-H+ was carried out by using a method similar to that described by Belbachir and al (39). In an Erlenmeyer flask, crushed raw Maghnite (30 g) was dispersed in a volume of distilled water (120 mL). The mixture was stirred using a magnetic stirrer for 2 h at room temperature. Then, a solution of sulfuric acid 0.5M (100 mL) was added. The solution thus obtained was maintained for two days under stirring, and then the mineral was filtered off and washed several times with distilled water up to pH 7. After filtration, the Mag-H+ was dried in an oven for 24 h, at 105°C and was then crushed.
Synthesis of poly (1,3-dioxolane) macromonomers :
Macromonomers are linear macromolecules carrying polymerizable functions at one or two chains ends (43,44). Numerous examples of macromonomer synthesis by different polymerization processes and extensive studies on their copolymerization behavior have been reported (45-48).
Bifunctional PDXL macromonomers are obtained by cationic ring opening polymerization of 1,3-dioxolane (DXL) in the bulk at 20°C, catalyzed by Mag-H+ in the presence of methacrylic anhydride(40). Amount of mag-H+ dried just before use for 1hour during oven 100°C and placed in a flask. DXL and methacrylic anhydride were added. The reaction mixture was stirred for 1hour under nitrogen. The product was dissolved in dichloromethane (CH2Cl2) and filtered to eliminate the Mag-H+. The dichloromethane was removed by evaporation, the polymer was redissolved in THF and the PDXL solution was precipitated in cold ethanol under stirring. The precipitated polymer was dried under vacuum.
The resulting PDXL macromonomers were characterized by RMN-H1 (BRUKER 300) (internal reference: tetramethylsilane) for average functionality determination of the different macromonomers. Size exclusion chromathograhy (SEC) using poly (tetraethylene oxide glycol) (POEG) samples as standard for calibration and THF as eluent, these SEC experiments are used to determine molar mass of the polymers. The double bond content was also obtained by UV spectroscopy (UV/visible shimadzu 2101 PC apparatus) in acetonitrile (λ=206nm, reference: ethylmethacrylate, ε = 8511).
The PDXL macromonomers obtained were fitted with methacrylate end groups (Scheme 1).
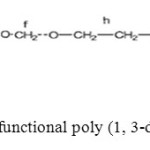 |
Scheme1: Structure of bifunctional poly (1, 3-dioxolane) macromonomer Click here to View scheme |
Polymerization of bifunctional PDXL macromonomers: Pure PXL hydrogel synthesis
Bifunctional PDXL macromonomers are constituted of hydrophilic chains fitted with polymerizable hydrophobic methyl methacrylate units at both chain ends. They behave typically as amphiphiles. Their solubility depends on one hand on the chemical nature of the solvent and on the other hand on the nature of the polymerizable units and the chain length. Goethals and al (49) took advantage of the amphiphilic character of PDXL macromonomers for prepared interpenetrating hydrogels with outstanding mechanical properties by copolymerization of PDXL macromonomers with methyl methacrylate (MMA). Naragui and al (48) used a similar approach to obtain PDXL hydrogels directly in water, two cases are considered: the preparation of pure PDXL networks and that of poly(ethylene oxide)(POE) hydrogels with short PDXL segments, in order to provide access to a new class of “intelligent” degradable hydrogels reactive to the environment. In the present work, we also attempted the free radical homopolymerization of bifunctional PDXL macromonomers in aqueous solution, using potassium persulfate (K2S2O8) as initiator at 60°C (2 mol% versus double bond content). Hydrogels were synthesized over a large range of concentration and for different molar masses of the macromonomer precursor chains. The crosslinking reaction is schematically represented on Scheme 2. The gel point was reached after a few hours and was dependent upon the chain length of the macromonomer precursor and its concentration. In order to reach the highest possible conversion (i.e. to incorporate as many chains as possible) the reaction was kept going for at least 24 hours. At the end of the polymerization, hydrogels products were immersed in excess distilled water and then filtered for several times to extract any soluble materials, i.e. precursor chains not connected to the hydrogel structure.
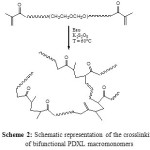 |
Scheme2: Schematic representation of the crosslinking of bifunctional PDXL macromonomers Click here to View scheme |
Preparation of maghnite-Na+
Maghnite-Na+ was prepared as follows: crushed raw maghnite (5g) was dispersed in (400ml) hexaphosphate sodium aqueous solution (1M) The mixture was stirred vigorously for 48 h, and then Mag-Na+ was formed. The separated Mag-Na+ was washed with large volume of distilled water and filtered several times until the excessive anionic entity was completed removed, and then dried in vacuum oven 100°C.
Synthesis of PDXL/Maghnite-Na+ hydrogels
PDXL/maghnite-Na+ hydrogels were prepared by free radical polymerization of the a,w-methacryloyloxy PDXL macromonomers in aqueous Mag-Na+ suspension using potassium persulfate as initiator at 60°C. Firstly, the appropriate amount of Mag-Na+ was suspended in water under stirring for 7 days (one week). Therefore, a homogeneous dispersion was observed. After that, the macromonomer was dissolved in suspended Mag-Na+ and taken in glass tubes of 2cm diameter. Finally, potassium persulfate was added to the solution in the tubes. The polymerization reaction was carried out for 24 hours at 60°C. The hydrogel product was purified in a similar manner to that of PDXL hydrogels.
Measurement of extractable amount
The solvent (water) was replaced every other day over a period of about two weeks until no further extractable polymer could be detected. The extractable amount of polymer e was determined by evaporating the solvent containing soluble materials (precursor chains not connected to the hydrogel structure), weighing the total residue. ε was obtained by dividing the weight of extractable amount by the weight of the polymer precursor.
ε (wt%) = weight extractable amount / weight polymer precurso
Measurement of equilibrium swelling
The hydrogels, free of linear unconnected chains, were characterized in terms of their swelling behavior according to well established procedures (50). The relationship to calculate the volume degree of equilibrium swelling (Qv)is from the measured weight degree of equilibrium swelling (G) of networks.
For the measurement of the weight degree of equilibrium swelling G, the swollen gel was placed in an oven at 100°C until the gel was dried. The weight degree of equilibrium swelling was obtained experimentally from the weight of the gel swollen to equilibrium and the weight of the same gel in the dry state.
G = (weight dry hydrogel + weight solvent) / (weight dry hydrogel)
The volume degree of equilibrium swelling Qv is calculated from G:
Qv = 1 + (G – 1) d0/ ds
ds and d0 are the densities of the solvent and the dry hydrogel, respectively.
Results and discussion
Characterization of bifunctional PDXL macromonomers:
Linear bifunctional PDXL macromonomer was prepared on one step by cationic polymerization, using Mag-H+ as initiator in the presence of methacrylic anhydride. The bifunctional PDXL macromonomers thus formed are constituted of hydrophilic chains fitted with polymerizable hydrophobic methyl methacrylate units at both chain ends. The methacrylate end groups are visible in the 1H-RMN spectrum of α,ω bis-unsaturated PDXL, as shown in figure 1.
According to the results presented in Table 1, we noted a few comments:
- When the amount of Mag-H+ used was 3 wt %, the highest rate of functionalization observed was around 87%. However, the use of a higher amount of Mag-H+ causes a certain lowering of the rate of functionalization, i.e., close to 65%. Apparently, because of its increased concentration, the catalyst attacks the acetal groups of PDXL, causing the destruction of some double bonds, as it has been demonstrated earlier (45, 48).
- The number average mass molar of PDXL synthesized with different amounts of catalyst, obtained by SEC, are remarkably constant, close to 5000 g.mol-1 for the range tested (3–10 wt % of Mag-H+).
 |
Figure1: 1H-RMN spectrum (300MHz) of bifunctional PDXL macromonomer, solvent: CDCl3 |
Table1: Molar characteristics of bifunctional PDXL macromonomer
| Reference |
MagH+(wt%) |
Mn a (SEC) |
Mn b (UV) |
Mwc(SEC) |
Mw/Mn(SEC)d |
Tf (%) |
| PDXL 1 |
3 |
4500 |
5200 |
7200 |
1.58 |
87 |
| PDXL 2 |
4 |
4550 |
— |
7300 |
1.60 |
— |
| PDXL 3 |
6 |
4600 |
7100 |
8400 |
1.82 |
65 |
| PDXL 4 |
10 |
5500 |
7800 |
8500 |
1.54 |
71 |
| PDXL 5 |
20 |
4500 |
7300 |
6900 |
1.53 |
62 |
All molecular weights are expressed in g moL_1.
a :Number average molar mass determined by SEC.
b :Number average molar mass determined by UV.
C: Mass average molar mass determined by SEC.
d: Polymolecularity
Tf: Amount of double bonds measured by UV (in acetonitrile).
Characterization of the hydrogels:
The hydrogels, free of soluble materials (i.e. species non-grafted to the hydrogel structure), were characterized by X-ray diffraction, Fourier transform infrared spectroscopy (FT-IR),p thermal analysis and their equilibrium swelling behavior in water.
X-ray diffraction
To investigate the structure of composites, the x-ray diffraction (XRD) was employed in this paper. The change of the basal spacing (d001) of clay patelets is the criterion by which the intercalated or exfoliated composites can be decided.
The XRD patterns of Mag-Na+ and poly(1,3-dioxolane)/Mag-Na+ hydrogel containing 3% wt of Mag-Na+ are shown in figure 2 and 3 respectively.
The XRD pattern of Mag-Na+ show two strong peaks at 5, 6° and 5, 7° corresponding to basal spacing of 15,77A° and 15,5A° respectively.
The XRD profile of poly(1,3-dioxolane)/Mag-Na+ hydrogel show a peak at 1.92° corresponding to the basal spacing of 46.02A°.
XRD measurement revealed that basal spacing was increased, so an intercalated or partially exfoliated morphology for the prepared poly(1,3-dioxolane)/Mag–Na+ hydrogel was obtained.
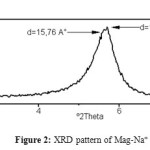 |
Figure2: XRD pattern of Mag-Na+ Click here to View figure |
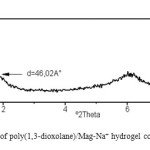 |
Figure3: XRD pattern of poly(1,3-dioxolane)/Mag-Na+ hydrogel containing 3% wt Mag-Na+ Click here to View figure |
FT-IR analysis
The FT-IR spectrum of Mag-Na+, pure PDXL hydrogel and PDXL/Mag-Na+ hydrogel containing 50% of PDXL and 3% of Mag-Na+, are shown in figure 4. Pure PDXL hydrogel and PDXL/Mag-Na+ hydrogel have characteristic bands of PDXL: 1010 cm-1 and 2880 cm-1 corresponding to the band of C-O-C and CH2—CH2 respectively. Besides these bonds, PDXL/Mag-Na+ hydrogel has also the characteristic bands of Mag-Na+:1000; 518; 461 cm-1 corresponding to the band of Si-O stretching and deformation respectively. After incorporated Mag-Na+ into PDXL network, the OH stretching of Mag-Na+ at 3600 disappeared; this may be according to the possibility of reaction between Mag-Na+ and PDXL macromonomers during the polymerization process. This indicates that PDXL chains stay immobilized inside or on the surface and edge of clay layers.
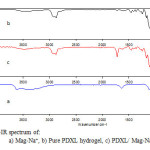 |
Figure4: FT-IR spectrum of:a) Mag-Na+, b) Pure PDXL hydrogel, c) PDXL/ Mag-Na+ hydrogel |
Thermal stability
Thermal stability is the ability of a material to maintain its physical properties when exposed to high temperatures. The thermogravimetric analysis (TGA) of pure PDXL hydrogel and the PDXL hydrogel containing 3% of Mag-Na+ was carried out. As shown in figure 5 and 6. The pure PDXL hydrogel exhibit a two stage thermal decomposition. The first, stage is in the range of 77-134°C due to a loss of moisture present in the sample. The second stage is in the range of 366-395°C attributed to the thermal decomposition of poly (1,3-dioxolane). As for PDXL/Mag-Na+ hydrogel, the major weight loss started at 372°C. Thus, the decomposition temperature of PDXL/Mag-Na+ hydrogel was higher than the pure PDXL hydrogel. This result indicates that the introduction of Mag-Na+ could improve the thermal stability.
This is an additional evidence that the montmorillonite layers were intercalated and dispersed in the polymer matrix, although with different grade of dispersion for Mag-Na+. The improvement of thermal stability can be attributed to the barrier effect of montmorillonite. Montmorillonite is a layered structure and small molecules generated during thermal decomposition process cannot permeate, and thus have to bypass, montmorillonite layers (51).
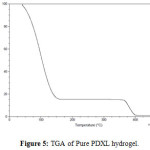 |
Figure5: TGA of Pure PDXL hydrogel. Click here to View figure |
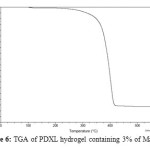 |
Figure6: TGA of PDXL hydrogel containing 3% of Mag-Na+ Click here to View figure |
Swelling measurement
Swelling behavior of PDXL hydrogels
Hydrogels were synthesized over a large range of concentration and for different molar masses of the precursor chains. According to the results reported on table 2, the following comments can be made:
- For a given molar mass, the values of the amount of extractable polymer and the volume degree of equilibrium swelling Qv measured in organic solvent (THF, CH2Cl2) or in water decreases with increasing concentration precursor (Figure 7). This demonstrates that the density of elastically effective chains is essentially influenced by the macromonomer precursor concentration. The presence of high concentration of polymerizable groups leading to an increase of the probability of reaction between polymerizable groups, so the network structure was compact and the swelling was less important. This result can easily be interpreted by the fact that in aqueous medium, the PDXL macromonomers (which contain a hydrophobic main chain but also a hydrophobic polymerizable end-group) are kept together by the aggregation of their hydrophobic ends (micellization). Similar results have been observed in previous publications(3,22,48) in the case of free radical homopolymerization of bifunctional PEO or PDXL macromonomers, when conducted in water at 60°C. The authors also observed a higher polymerization rate that they explained through the same phenomenon of micellization.
- Whatever the swelling solvent (water, THF or dichloromethane), the values of the volume degree of equilibrium swelling measured in water are higher than those measured in THF or dichloromethane. This is attributed to better solubility of PDXL chains in water than in THF (or in dichloromethane). PDXL solutions in water are characterized by the presence of aggregates (52). For PDXL networks swollen in water, the presence of aggregates should be translated into additional crosslinking points. Similar results have been observed on PDXL networks synthesized by endlinking and involving long PDXL chains (53), such networks swell more in water than in dioxane. The authors explained this result by the hydrophobic nature of the urethane linkages. Below a given volume fraction of polymer these junction points tend to aggregate, inducing heterogeneities in the network. For macromonomer based homopolymeric PEO or PDXL hydrogels, especially for those characterized by long precursor chains, i.e with low contents of hydrophobic core, volume degree of equilibrium swelling are higher in water than in organic solvents (48).
- The volume degree of equilibrium swelling of hydrogel Qv was dependent upon the length of the macromonomer precursor chains. The networks formed with short PDXL chain segment should have a higher crosslinking density and compact structure, so that, the solvent can hardly permeate into the networks and lead to a low degree of swelling. The networks with long linear chains can lead to network with a loose structure; therefore, solvent can easily permeate into the network and the degree of swelling was higher.
Table2: Physico-chemical characteristics of Pure PDXL hydrogel prepared in water at 60°C over 24h with K2S2O8 as initiator
|
Reference |
Mn a |
[PDXL] (mol.L-1) b
|
e (wt%) c |
Qv water d |
Qv THF e |
Qv CH2Cl2 f |
|
PDXL6 |
3000 |
0,13 |
0,66 |
20,35 |
12,17 |
22,34 |
|
PDXL7 |
3000 |
0,20 |
1,00 |
19,06 |
12,66 |
21,89 |
|
PDXL8 |
4000 |
0,38 |
1,60 |
11,32 |
– |
– |
|
PDXL9 |
4000 |
0,22 |
2,13 |
17.77 |
10.62 |
16.84 |
|
PDXL10 |
4000 |
0,17 |
2,20 |
22.93 |
13.02 |
20.21 |
|
PDXL11 |
4000 |
0,13 |
2,26 |
28.09 |
13.32 |
23.63 |
|
PDXL12 |
4000 |
0,06 |
4,60 |
31.96 |
14.32 |
27.19 |
|
PDXL13 |
5500 |
0,13 |
2,36 |
29,00 |
21,41 |
27,19 |
a : Number average molar mass determined by SEC, expressed in g moL_1.
b :Concentration of precursor chains in mol.L-1.
c :Amount of extractible polymer in wt% after 48h
d : Qv water: the volume degree of equilibrium swelling measured in water
e : Qv THF: the volume degree of equilibrium swelling measured in THF
f : Qv CH2Cl2 : the volume degree of equilibrium swelling measured in CH2Cl2
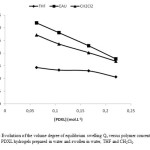 |
Figure7: Evolution of the volume degree of equilibrium swelling Qv versus polymer concentration of Pure PDXL hydrogels prepared in water and swollen in water, THF and CH2Cl2. |
Swelling behavior of PDXL/ Mag-Na+ hydogel:
The effect of the content of Mag/Na+ on the volume degree of equilibrium swelling Qv of PDXL/Mag-Na+ hydogels:
Hydrophilic sodium Montmorillonite clay: Maghnite-Na+ played an important role during the process of PDXL/ Mag-Na+ hydrogel synthesis. In this paper, the content of MMT-Na+(Mag-Na+) influences the swellability of hydrogels seriously. The results are shown in Figure 8. The volume degree of equilibrium swelling Qv measured in distilled water of hydrogel was the highest in the range of 3~7 wt% of Mag-Na+. Outside of the range, the volume degree of equilibrium swelling Qv of PDXL/ Mag-Na+ hydrogel decreased. This may be attributed to the fact that the clay could react with PDXL and acts as crosslink points in the corresponding hydrogel and also, this is because in the PDXL/ Mag-Na+ hydrogel, the hydrophilic sodium MMT layers were disperses in the polymer matrix on the nano-scale, this results in the strong interaction between hydrophilic sodium MMT layers and polymer chains and the degree of crosslinking was higher, therefore, degree of swelling is lower.
The formation of new chemical and physical bonds in the polymer network representing new crosslinking points, leads to a decrease in the water absorption capacity of the synthesized hydrogel nanocomposites. A similar result was obtained with the majority of hydrogel nanocomposite based on polymer water soluble (54-58).
When the content of Mag-Na+ was 5 wt%, the maximum of the volume degree of equilibrium swelling Qv is observed. At low MMT-Na+ concentrations the MMT-Na+ layers were well separated, making the negative surface charges accessible for both the functional groups of the polymer and the incoming water molecules, then hydrogel swell better. Similar results (59) have been observed for a series of hydrophilic and hydrophobic polymer hydrogel containing hydrophilic sodium Montmorillonite (MMT-Na+), when addition of hydrophilic montmorillonite particles improved swelling of the gel only when applied at low concentration (1-5 wt. %).
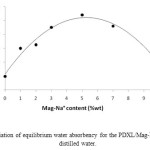 |
Figure8: variation of equilibrium water absorbency for the PDXL/Mag-Na+ hydrogel in distilled water. Click here to View figure |
The effect of the concentration of macomonomer precursor on the volume degree of equilibrium swelling Qv of PDXL/Mag-Na+ hydogels:
To further investigate the influence of the concentration of macomonomer precursor PDXL on the volume degree of equilibrium swelling Qv of PDXL/Mag-Na+ hydogels, a series of experiments (content of hydrophilic sodium MMT: Mag-Na+ was 5 wt%, other conditions were kept constant) were carried out. From the results shown in Table 3, an interest tendency could be observed: When the concentration of macromonomer precursor is 0,075 mol/L, the volume degree of equilibrium swelling measured in water of PDXL/Mag-Na+ hydogels had the highest value of 25,51.
However, whether the concentration of macromonomer precursor increased, the volume or weight degree of equilibrium swelling of hydrogels all decreased.
The addition of Mag-Na+ particles enhanced the cross-linking density of hydrogel, especially at high MMT-Na+ concentrations. This is because in the PDXL/Mag-Na+ hydogels, the montmorillonite layers were dispersed in the polymer matrix on the nano-scale, this results in the strong interaction between montmorillonite layers and polymer chains and the degree of cross-linking was higher, therefore, degree of swelling is lower. However, with increasing of the concentration of PDXL macromonomer, the hydrophilicity of the nanocomposites decrease, which impairs the swelling capacity of composites.
It was also found, that the volume degree of equilibrium swelling measured in water of the pure PDXL hydrogels was higher than that of those PDXL/Mag-Na+ hydogels as shown in Figure 9.
Thus, the volume degree of equilibrium swelling Qv of hydrogels changed with the level of sodium MMT. This is attributed to the stronger affinity of the pure PDXL hydrogels for water than of PDXL/Mag-Na+ hydogels.
Table3: Physico-chemical characteristics of PDXL/Mag-Na+ hydrogels prepared in water at 60°C over 24h with K2S2O8 as initiator.
|
Reference |
[PDXL] (mol.L-1) a
|
G water c |
Qv water d |
|
PDXLNa1 |
0,075 |
20 |
25,51 |
|
PDXLNa2 |
0,125 |
17,33 |
22,06 |
|
PDXLNa3 |
0,175 |
10,94 |
13,82 |
|
PDXLNa4 |
0,225 |
10,12 |
12,76 |
Mn =4000 g moL_1:Number average molar mass determined by SEC.
Content of hydrophilic sodium MMT: Mag-Na+ was 5 wt%,
a: Concentration of precursor chains in mol.L-1.
b: Gwater: The weight degree of equilibrium swelling measured in water.
c: Qv water: the volume degree of equilibrium swelling measured in water
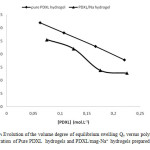 |
Figure9: Evolution of the volume degree of equilibrium swelling Qv versus polymer concentration of Pure PDXL hydrogels and PDXL/mag-Na+ hydrogels prepared in water. Click here to View figure |
Conclusion
Telechelic poly (1,3-dioxolane) with methacrylate end groups have been synthesized on one step by cationic ring opening polymerization of 1,3-dioxolane, in the presence of methacrylic anhydride, using Mag-H+ as catalyst.
Hydrogels composed of poly(1,3-dioxolane) were obtained by free radical polymerization of well-defined bifunctional PDXL macromonomers in aqueous solution and in aqueous Mag-Na+ suspension respectively. The XRD ray FT-IR was employed to investigate the structure. It suggested that the polymer chains were grafted on to the surface and edge of clay particles during polymerization.
At the same time, their swelling properties were investigated. The volume degree of equilibrium swelling of the pure PDXL hydrogels and PDXL/Mag-Na+ hydogels was investigated as a function of the concentration of macromonomer precursor and the content of clay particles.
The following conclusions can be drawn:
- By increasing polymer precursor concentration, the structure of the hydrogel was compact and the swelling was lower.
- The volume degree of equilibrium swelling in water of PDXL/Mag-Na+ hydogels decreases with increasing Mag-Na+ content owing to the increasing cross-linking point and the decreasing percentage of hydrophilic groups in polymeric hydrogel.
- The volume degree of equilibrium swelling measured in water of the pure PDXL hydrogels was higher than that of those PDXL/Mag-Na+ hydogels. This is attributed to the stronger affinity of the pure PDXL hydrogels for water than that of PDXL/Mag-Na+ hydogels.
- The results showed that the performances of hydrogels were improved by the introduction of clay particles.
- The disperse stability of clay and the polymerization between of sodium MMT and PDXL macromonomer should be considered as the most important factors, which could seriously influence the equilibrium swelling of hydrogels in water.
- Finally, the result of TGA suggested that the introduction of Mag-Na+ could improve the thermal stability.
References
- Graham. N. B, , Peppas. N.A, ‘‘Hydrogels in Medicine and Pharmacy’’, Ed.CRC Press, Boca Raton 1987.
- Peppas. N. A, Bures. P, W. Leobandung, H. Ichikawa ,Eur. J.Pharmac. Biopharmac.2000, 50, 27.
- Lutz. P. J,Macromol. Symp. 2001, 164-277.
- Lutolf . M. P, Reaber. G. P, Zisch. A. H, Tirelli. N, Hubbell. J. A, Adv. Mater 2003, 15, 888.
- Haque, M.A., Kurokawa, T., Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer. 2012, 53 (9), 1805–1822.
- Khetani, S.R., Bhatia, S.N., Engineering tissues for in vitro applications. Curr. Opin.Biotechnol. 2006, 17, 524–531
- Keshava Murthy, P.S., Murali Mohan, Y., Sreeramulu, J., Mohana Raju, K., React. Funct. Polym. 2006. 66, 1482–1493.
- Benoit, D.S.V., Nuttelman, C.R., Collins, S.D, Anseth, K.S., Biomaterials, 2006, 27, 6102–6110.
- Hervas Perez, J.P., Lopez-Cabarcos, E., Lopez-Ruiz, B, Biomol Eng. 2006. 23, 233–245
- Buchhloz. F.L, Graham.T, Modern Superabsorbent Polymer Technology, Wiley-VCH, New York, 1986, p. 1 and 152.
- Jin, M., Zhong, Q.,. Structure modification of montmorillonite nanoclay by surface coating with soy protein. J. Agric. Food Chem. 2012, 60 (48), 11965–11971.
- Buchhloz. F.L, Peppas. N. A (Eds), Superabsorbent polymer science and technology, ACS Symposium Series, 1994, Vol. 573, p. 121.
- Sun. X, Shi. G, Tang. B, Wu. Z, J. Appl. Polym. Sci. 2002, 86, 3712.
- Ende. M, Hariharan. D, Pappas. N.A, React. Polym. 1995, 25, 127.
- Dorkoosh. F.A, Brusee. J.C, Verhoef, G. Borchard, Tehrani. M.R, Junginger. H.E, Polymer, 2000, 41, 8213.
- Dzionmwa. G.P.T, Wood. C.J, Hill. D.J.T, Polym. Adv. Technol. 1997, 8,762.
- Spagnol, C., Rodrigues, F.H., Neto, A.G., Pereira, A.G., Fajardo, A.R., Radovanovic, E., Rubira, A.F., Muniz, E.C.,. Nanocomposites based on poly(acrylamide-co-acrylate) and cellulose nanowhiskers. Eur. Polym. J. 2012, 48 (3), 454–463.
- Griffith Cima. L, Lopina. S. T, Macromolecules 1995, 28,787.
- Gnanou. Y, Hild .G, P. Rempp, Macromolecules 1984, 17,945.
- Rempp. P, Lutz. P. J, Masson. P, Franta. E, Makromol. Chem. Suppl. 1984, 8, 3.
- Schmitt .B, Alexandre. E, Boudjema. K, Lutz. P. J, Macromol. Symp. 1995, 93, 117.
- Schmitt. B, Alexandre. E, Boudjema. K, Lutz .P. J, Macromol. Biosci. 2002, 2, 341.
- Kabiri K, Zohuriaan-Mehr MJ. Polym Adv Technol 2003, 14, 438.
- Li A, Wang A, Chen J. J Appl Polym Sci 2004, 92, 1596.
- Baït, N., Grassl, B., Derail, C., Benaboura, A. Hydrogel nanocomposites as pressuresensitive adhesives for skin-contact applications. Soft Matter 2011, 7 (5), 2025–2032.
- Ikeda, M., Yoshii, T., Matsui, T., Tanida, T., Komatsu, H., Hamachi, I.,. Montmorillonite-supramolecular hydrogel hybrid for fluorocolorimetric sensing of polyamines. J. Am. Chem. Soc. 2011, 133 (6), 1670–1673.
- Taki, A., Baiju, J., Arakawa, S., Okamoto, M.,. Structure and rheology of nanocomposite hydrogels composed of DNA and clay. Eur. Polym. J. 2013, 49 (4), 923–931.
- Ma, J., Xu, Y., Fan, B., Liang, B.,. Preparation and characterization of sodium carboxymethylcellulose/poly(N-isopropylacrylamide)/clay semi-IPN nanocomposite hydrogels. Eur. Polym. J. 2007, 43 (6), 2221–2228.
- Bao, Y., Ma, J., Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84 (1), 76–82.
- Irani, M., Ismail, H., Ahmad, Z.,. Preparation and properties of linear low density polyethylene-g-poly(acrylic acid)/organo-montmorillonite superabsorbent hydrogel composites. Polym. Test. 2013, 32 (3), 502–512.
- Santiago F, Mucientes AE, Osorio M, Pobleteb JP, Polym Int. 2006. 55: 1-9.
- Liu PS, Li L, Zhou NL, Zhang J, Wei SH, Shen J. J Appl Polym Sci 2006, 102: 5725-5730.
- Qingsong Zhang, Xuewei Li, Yiping Zhao, Li Chen, Preparation and performance of nanocomposite hydrogels based on different clay. Applied Clay Science 2009, 46, 346–350
- Lin J, Wu J, Yang Z, Pu. M. Macromol Rapid Commun 2001. 22:422-424.
- Anamaria Zaharia , Andrei Sarbu , Anita-Laura Radu , Katja Jankova , Anders Daugaard, Søren Hvilsted François-Xavier Perrin , Mircea Teodorescu , Cornel Munteanu , Victor Fruth-Oprisan . Preparation and characterization of polyacrylamide-modified kaolinite containing poly [acrylic acid-co-methylene bisacrylamide] nanocomposite hydrogels. Applied Clay Science, 2015, 103, 46–54
- Lee WF, Chen YC .J Appl Polym Sci 2005, 97:855-861.
- Zhang J, Wang A Study on superabsorbent composites. IX: synthesis, characterization and swelling behaviors of polyacrylamide/clay composites based on various clays. React Funct Polym, 2007, 67 (8):737–745.
- Paranhos CM, Soares BG, Oliveira RN, Pessa LA. Macromol Mater Eng 2007, 292:620-626.
- Belbachir.M and Bensaoula.A; composition and method for catalysis using bentonites; U.S. Patent N° 7,094,823; Aug, 22, 2006.
- Reguieg.F, Sahli.N, Belbachir. M, Lutz. P. J. Journal of Applied Polymer Science, 2006, 99, 3147-3152.
- Benadda.M, Ferrahi. M. I, Sahli.N, Belbachir.M. The Open Catalysis Journal, 2009, 2, 166-168.
- Beloufa.K, Sahli.N, Belbachir.M, Journal of Applied Polymer Science, 2010, 115, 2820–2827.
- Rempp, P.; Franta, E. Adv Polym Sci 1984, 1, 58.
- Chujo, Y.; Yamashita, Y. In Telechelic Polymers: Synthesis and Application; Goethals, E. J., Ed.; CRC: Boca-Raton, FL, 1989; p163.
- De Clercq, R. R.; Goethals, E. J. Macromolecules 1992, 25, 1109.
- Rempp, P.; Gnanou, Y. Macromol Chem Phys 1987, 188, 2111.
- Sahli. N.; Ould Kada. S.; Belbachir. M; Franta.E; Lutz.P. J;Reibel. L. Macromol Symp 1994, 85, 167.
- Naraghi, K.; Sahli, N.; Belbachir, M.; Franta, E.; Lutz, P. J. Polym Int 2002, 51, 912
- Goethals. E, De Clerq. R. R, Saskia. W,J. Macromol. Sci.,Pure Appl. Chem.1993, A30, 679.
- Hild. G, Prog. Polym. Sci. 1998, 23, 1019.
- Liu Z, Zhou P, Yan D. J Appl Polym Sci 2004; 91:1834.
- Benkhira. A, Franta. E, Raviso. M, Francois. J, Macromolecules, 1994, 27, 3963.
- Gérard. E, Gnanou. Y, Rempp. P, Macromolecules, 1990, 23, 4299.
- Paranhos CM, Soares BG, Oliveira RN, Pessan LA. Poly(vinyl alcohol)/clay-based nanocomposite hydrogels: Swelling behavior and characterization. Macromol Mater Eng 2007 292(5):620–626.
- Gholam Reza Mahdavinia & Bakhshali Massoumi & Karim Jalili & Gholamreza Kiani, Effect of sodium montmorillonite nanoclay on the water absorbency and cationic dye removal of carrageenan-based nanocomposite superabsorbents. J Polym Res, 2012 19:9947
- Elbadawy A. Kamoun & Henning Menzel, HES-HEMA nanocomposite polymer hydrogels: swelling behavior and characterization, J Polym Res 2012, 19:9851
- Gholam Reza Mahdavinia , Javad Hasanpour, Zeinab Rahmani, Shiva Karami, Hossein Etemadi, Nanocomposite hydrogel from grafting of acrylamide onto HPMC using sodium montmorillonite nanoclay and removal of crystal violet dye. Cellulose, 2013, 20:2591–2604.
- Xudong Nie, Abudurman Adalati, Juan Du, Huanhuan Liu, Shimei Xu, Jide Wang. Preparation of amphoteric nanocomposite hydrogels based on exfoliation of montmorillonite via in-situ intercalative polymerization of hydrophilic cationic and anionic monomers. Applied Clay Science, 2014, 97–98 132–137
- Laslo. J, Janos. V, Lajos K, Imre. D, Applied clay science, 2009, 43, 260–270.

This work is licensed under a Creative Commons Attribution 4.0 International License.









