Synthesis of pure zeolite P2 from calcium silicate hydrate; tobermorite
Nasser Y. Mostafa1,2*, Rasha A. Garib1,2, Z. K. Heiba1,3, Omar H. Abd-Elkader4,5 , M. M. Al-Majthoub1
1Faculty of Science, Taif University, P.O. Box: 888, Al-Haweiah, Taif, Saudi Arabia.
2Chemistry Department, Faculty of Science, Suez Canal University, Ismailia 41522, Egypt.
3Physics Department, Faculty of Science, Ain Shams University, Cairo, Egypt.
4Electron Microscope Unit, Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
5Electron Microscope & Thin Films Department, Physics Division, National Research Center, Dokki 12622, Cairo, Egypt.
E-mail address: nmost69@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310254
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2015
Calcium silicate hydrate phases offer the possibility to become potential zeolites precursors due to its high silica contents. Pure calcium silicate hydrate phase; tobermorite (Ca5Si6O16(OH)2·4H2O), was prepared by hydrothermal method at 175°C. Tobermorite was sucssefully converted to Zeolite P2 for the first time via refluxing in 3 M NaOH solution and in the presence of Al source. Sodium hydroxide removed calcium ions from the interlayers of calcium silicate phase and form mesoporous zeolite. The pure zeolite was obtained after extraction of Ca(OH)2 with sugar solution. The zeolite products were characterized by using X-ray diffraction spectroscopy (XRD) and Scanning Electron Microscopy (SEM) with microanalysis (EDX). The Si/Al molar ratio of zeolite P can be controlled by vering the initinal Si/Al molar ratio. The cation-exchange capacity (CEC) of the produced zeolite P was higher than those produced from fly ash.
KEYWORDS:Zeolite P; calcium silicate hydrate; Hydrothermal; Zeolite synthesis
Download this article as:| Copy the following to cite this article: Mostafa N. Y, Garib R. A, Heiba Z. K, Abd-Elkader O. H, Al-Majthoub M. M. Synthesis of pure zeolite P2 from calcium silicate hydrate; tobermorite. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Mostafa N. Y, Garib R. A, Heiba Z. K, Abd-Elkader O. H, Al-Majthoub M. M. Synthesis of pure zeolite P2 from calcium silicate hydrate; tobermorite. Available from: http://www.orientjchem.org/?p=9008 |
Introduction
Semicrytalline calcium silicate hydrates (CSH) are the main components of cement hydration products. The CSH phases are metastable and transform to stable well crystalline phases like tobermorite, xontolite and gyrolite with hydrothermal treatments [1-3]. Tobermorite, Ca5Si6O16(OH)2·4H2O has many industrial applications include; non-asbestos heat insulations [4, 5], adsorbents for organic or inorganic effluents [6], radioactive waste stabilization and cation exchangers [7-10]. Tobermorite, generally occurs in two varieties; normal and anomalous. The difference between the normal and anomalous types arises from the difference between their thermal behaviors [11]. Both varieties lose interlayer water upon heating to 300 °C, but the lattice of normal type shrinks from 11.3 to 9.5 nm. The anomalous variety does not shrink. Both types of tobermorite consist of layers of Ca–O bonded to chains of silica tetrahedra running along the b axis [12]. In anomalous tobermorite, the bridging tetrahedra in one chain are bonded to those in the chain across the interlayer region [13, 14]. The limiting composition for normal tobermorite is about Ca5Si6O16(OH)2·4H2O (Ca/Si = 0.91)[15].
Many researchers [16-19] have investigated the conversion of industrial by-products to zeolite. Fly ashs that contain high amount of amorphous phases are the most extensively studied waste for zeolite synthesis. Variety of zeolite types include zeolite A [20], phillipsite and faujasite and GIS-type zeolites [16, 19] have been synthesis from flay ashs. However, until now, producing of pure well crystalline zeolite from the wastes was still a challenging task. The structure of the silica source has dominante effect on zeolitzation process [21]. Many researchers prepared zeolite P from natural silica sources [22-24] or by-products such as fly ash [20, 25, 26]. The small pore size of zeolite P makes it suitable for subtraction of toxic and radioactive ions [27]. Zeolite P exhibits a higher capacity for adsorbing Ca2+ and Mg2+, thus it used also as of a detergent additive.
In this paper, we report the synthesis and characterization of zeolite P from calcium silicate hydrate phase as precursor. This synthesis route is simple and gives high yield of zeolite P. Furthermore, the productd zeolites have high purity with controlled Al/Si ration.
Materials and Methods
Calcium Silicate Prepration
CaO was prepared by heating pure grade calcium carbonate at 1100 °C for 3 h. Min-U-Sil silica is used as silica source (10 μm). All other chemicals are analytical grade. For the preparation of tobermorite, calcium oxide (CaO) and silica, were used as starting materials. The starting materials with batch compositions of Ca/Si = 0.83 were added to boiled deionized water, quickly loaded into the autoclave. Teflon-lined steel autoclave 350 cm3 capacity equipped with magnetic stirrer and heat controller, was used in the preparation. The hydrothermal syntheses were done using a water/solid ratio of 10 at 175 °C for the curing times of 24 h. After the hydrothermal treatment, the samples were separated by filtration, washed repeatedly with deionized water, and then dried at 105 °C for 24 h.
Zeolite P Synthesis
Sodium meta-aluminate was prepared using 15 g of NaOH powder, 15 g of Al(OH)3 powder, and 20 ml of deionized water mixed in a glass beaker at 100 °C for 1 h. A proper amount of deionized water was added to dilute the above sodium meta-aluminate solution to 100 ml. one gram of tobermorite and 100 ml of 3.0 M NaOH solution were first mixed in a 250-mL polyethene bottle, and the bottle was placed in a water bath at 95 °C. Sodium meta-aluminte solution was added dropwise to the bottle with continuous stirring. After reaction for 72 h and subsequent filtering, washing with deionized water, and drying at 100 °C, the product zeolite P was obtained. The solid product was washed thoroughly with 10% sugar solution then with distilled water, dried in air at 100 °C for 12 h. The crystal structure and purity of the obtained products were analyzed by using XRD, whereas textural structures were examined by using Scanning Electron Microscope (SEM: JEOL, with Au-coated, operated at 15 keV).
Cations Exchange Capacities (CEC)
The cation-exchange capacity (CEC) of the produced zeolite P and tobermorite were characterized and compared to that of the commercial grade zeolite P. Cation exchange capacities, CEC (meq/100 g) for synthesized samples were measured by using method as described by Komarneni and Roy [30,31]. Procedures were conducted in triplicate for each sample. Approximately 50mg of accurately weighed sample were equilibrated with 1.0 M KCl solution to saturate the exchange sites with K+ ions. The samples received three further washings with 0.01 KCl solution to remove the excess of KCl and to prevent hydrolysis before being centrifuged. The samples were then equilibrated with four consecutive washings with 1M CsCl solution to displace the K+ ions from the exchange sites. The four 1M CsCl solution washings were combined and analysed for K+ using inductively coupled plasma atomic emission spectrometer (ICP-OES) model Perkin-Elmer Optima 2100DV. The CEC was estimated from the ratio of the amount of displaced K+ ions to the original sample mass.
Characterization
X-ray diffraction patterns (XRD) were obtained using Bruker diffractometer D8-advance with Cu-Kα radiation and Ni filter. The operating voltage and current were 35 kV and 35 mA, respectively. The samples morphology was investigated using SEM (JOEL, Model: JSM-5600, Japan.) equipped with secondary electron detector and EDX.
Results and Discussion
Tobermrite synthesis and characterization:
XRD pattern of tobermorite sample produced by hydrothermal curing at 175 °C for 24 h are shown in Fig. 1. The product was pure tobermorite (JCPDS No. 01-088-1328) with no detected quartz or Ca(OH)2 peaks. The sharp and strong basal spacing peak at 1.1 nm corresponds the diffraction of 002 planes. This peak indicated perfection in the basial spacing between silicate layers [12]. This results is in agreement with our previous study [3]. Also this may attributed to the preferred orientation along this crystal plane. The preferred orientation results from pressing the powders with plat-like morphology in XRD holder. The main differences between the current study and previous studies [3, 12] are the purity and the CaO/SiO2 ratio of the starting mixes.
 |
Figure1: XRD pattern of tobermorite produced by hydrothermal treatment at 175 °C for 24 h. Click here to View figure |
SEM micrograph of tobermorite sample produced by hydrothermal curing for 24 h are shown in Figs. 2. Pure tobermorite (Fig. 2(a)) exhibited small platy crystallites, which are the typical morphology tobermorite [12]. The chemical composition of the tobermorite sample was determined by EDX and is shown in Fig. 2 b. The EDX spectra show the presence of elements Ca, Si and O without impurities. Quantitative analysis of the EDX spectra showed nearly the same Ca/Si ratio (0.83) as the stating mix, which confirms the complete conversion of the reacted CaO and SiO2 to pure tobermorite phase.
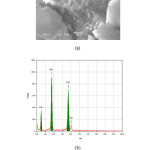 |
Figure2: (a) SEM images and (b) EDX spectra of tobermorite produced by hydrothermal treatment at 175 °C for 24 h. Click here to View figure |
Synthesis of Zeolite P
Adjusting the ratio of Si/Al in the reaction mixture prior to undergoing crystallization is the most important factor that determins the type of the end zeolite product. During preliminary trials, it was found that the Si/Al molar ratio of 3 was a critical ratio to prepare pure zeolite P. Using Si/Al ratio lower than 3, a mixture of zeolite P and amorphous silica was formed. Sodium aluminate solution was added to adjust the Si/Al ratio to 3 in the present investigation.
Fig. 3 shows the XRD pattern of synthesized zeolite after purification from Ca(OH)2. The figure confirms that high purity of zeolite P can be prepared from the reaction mixture with proper Si/Al ratio. The XRD lines could be well indexed to the orthorhombic (Space group: Pnma) structure of zeolite P2 (JCPDS No. 01-080-0700). Na4( Al4Si12O32 )( H2O )14
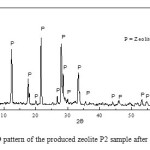 |
Figure3: XRD pattern of the produced zeolite P2 sample after purification. |
Fig. 4 shows SEM micrograph and corresponding EDX spectra of the zeolite P after washing with sugar solution. The SEM micrograph clearly indicating that no plat-like tobermorite particles were present. The morphology of the sample shows irregularly shaped paricles with size from 15 to 40 μm. Similar morphologies with different particle sizes (about 2- 6 μm) of zeolite P particles were reported by many investigators [28-30]. EDX spectra shows the presence of the elements O, Na, Si, and Al in the zeolite P samples and the Si/Al atomic ratio was 2.84 for the zeolite P sample obtained after washing with sugar solution.
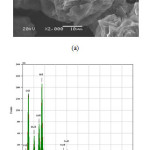 |
Figure4: (a) SEM images and (b) EDX spectra of the zeolite P2 sample after purification Click here to View figure |
Cation-Exchange Capacity
Results of cations exchange capacities (CEC) of the synthesized tobermorite and zeolite P are shown in Table 1. It was observed that tobermorite reveals low CEC value 0.35 meq/g. This value is very close to our pervious result [3]. Converstion of tobermorite to zeolite P increases CEC value to 3.89 meq/g. It is clear that the CEC values of zeolite P products synthesized from tobermorite was extremely high (3.79 meq/g) as compared to that of the orginal tobermorite sample (0.35 meq/g). It is also observed that the CEC values of the produced zeolite P are higher than zeolite P produced from fly ash (2.70 meq/g) [31].
Table1: Cation exchange capacity (CEC) of tobermorite, zeolite P and zolite P produced from fly ash [31].
| Sample | CEC (meq/g) |
| Tobermorite | 0.37 |
| Zeolite P | 3.79 |
| Zeolite P (from fly ash) | 2.70 [31] |
Conclusions
In this study, a clean and energy saving synthesis route was successfully applied for high purity zeolite P synthesis from low-value raw materials. Calcium silicate hydrates (tobermorite) was firstly hydrothermally prepared from quartz and calcium oxide at 175 °C for 24 h. Zeolite P have been prepared from calcium silicate hydrate phases as the silica source by employing alkali treatment at 95 °C with addition of aluminum source. Sodium hydroxide removes calcium ions from the interlayer space of tobermorite structure. Zeolite P was purified from the residue Ca(OH)2 by washing with sugar solution. The prepared zeolites P have ion exchange capacity (CEC) comparable with the commercial product. It was found that zeolite P has relatively high CEC values as 3.79 meq/g compared to 2.70 meq/g of zeolite P produced from fly ash.
Acknowledgements
This work was supported by Grants from Taif University, Saudi Arabia under project Grants No. 2485-434-1.
References
- Nakahira, A.; Naganuma, H.; Kubo, T.; Yamasaki, Y. J. Ceram. Soc. Jpn. 2008, 116, 500-504
- Shaw, S.; Clark, S. M.; Henderson, C. M. B. Chem. Geol. 2000, 167, 129-140
- Mostafa, N. Y.; Kishar, E. A.; Abo-El-Enein, S. A. J. Alloys Compd. 2009, 473, 538-542
- Heße, M. Keramische Zeitschrift 2011, 63, 190-192
- Mostafa, N. Y. Cem. Concr. Res. 2005, 35, 1349-1357
- Kaneco, S.; Itoh, K.; Katsumata, H.; Suzuki, T.; Masuyama, K.; Funasaka, K., et al. Environ. Sci. Technol. 2003, 37, 1448-1451
- Li, L.; Wu, Z. X.; Li, Y. X.; Sha, H. L.; Song, G. W. Desalin. Water Treat. 2013, 52, 4292-4297
- Coleman, N. J.; Bishop, A. H.; Booth, S. E.; Nicholson, J. W. J. Eur. Ceram. Soc. 2009, 29, 1109-1117
- Coleman, N. J.; Brassington, D. S.; Raza, A.; Mendham, A. P. Waste Manage. 2006, 26, 260-267
- Komarneni, S.; Roy, R.; Roy, D. M. Cem. Concr. Res. 1986, 16, 47-58
- Kikuma, J.; Tsunashima, M.; Ishikawa, T.; Matsuno, S. Y.; Ogawa, A.; Matsui, K., et al. Journal of Synchrotron Radiation 2009, 16, 683-686
- Mostafa, N. Y.; Shaltout, A. A.; Omar, H.; Abo-El-Enein, S. A. J. Alloys Compd. 2009, 467, 332-337
- Hartmann, A.; Buhl, J. C.; van Breugel, K. Cem. Concr. Res. 2007, 37, 21-31
- Hartmann, A.; Khakhutov, M.; Buhl, J. C. Mater. Res. Bull. 2014, 51, 389-396
- Jauberthie, R.; Temimi, M.; Laquerbe, M. Cem. Concr. Res. 1996, 26, 1335-1339
- Stamboliev, C.; Scopova, N.; Bergk, K. H.; Porsch, M. Stud. Surf. Sci. Catal. 1985, 24, 155-160
- Tamura, C.; Matsuda, M.; Miyake, M. J. Ceram. Soc. Jpn. 2006, 114, 205-209
- Vadapalli, V. R. K.; Gitari, W. M.; Ellendt, A.; Petrik, L. F.; Balfour, G. S. Afr. J. Sci. 2010, 106,
- Wang, Y.; Wang, L.; Xu, H.; Luo, S. Key. Eng. Mat. 2014, 591, 126-129
- Musyoka, N. M.; Petrik, L.; Hums, E. Adva.Mat.Res. 2012, 512-515, 1757-1762
- Bellussi, G.; Carati, A.; Rizzo, C.; Millini, R. Catal. Sci. Technolog. 2013, 3, 833-857
- Meftah, M.; Oueslati, W.; Ben Haj Amara, A. Physics Procedia 2009, 2, 1081-1086
- Meftah, M.; Oueslati, W.; Ben Haj Amara, A. IOP Conf. Ser. Mater. Sci. Eng. 2010, 13,
- Novembre, D.; Di Sabatino, B.; Gimeno, D.; Pace, C. Clay Miner. 2011, 46, 339-354
- Kurama, H. Glob. Symp. Recyc. Waste Treat. Clean Techn. 2005, 1319-1328
- Miyake, M.; Tamura, C.; Matsuda, M. J. Am. Ceram. Soc. 2002, 85, 1873-1875
- Atkins, M.; Glasser, F. P.; Jack, J. J. Waste Manage. 1995, 15, 127-135
- Kazemian, H.; Modarres, H.; Mobtaker, H. G. J. Radioanal. Nucl. Chem. 2003, 258, 551-556
- Subotić, B.; Šmit, I.; Madžija, O.; Sekovanić, L. Zeolites 1982, 2, 135-142
- Zhang, C.; Chen, N.; Zhang, X.; Zhan, F. Key Eng. Mater. 2014, 602-603, 71-75
- Querol, X.; Moreno, N.; Umaña, J. C.; Alastuey, A.; Hernández, E.; López-Soler, A., et al. Int. J. Coal Geol. 2002, 50, 413-423

This work is licensed under a Creative Commons Attribution 4.0 International License.









