Synthesis, Characterization Studies and Bond Valence Sum (BVS) analysis on 2:1 Adducts involving Zinc(II)dithiocarbamates and 4,4’-Bipyridine
Arumugam Manohar1*, Kuppukannu Ramalingam2 and Kottamalai Karpagavel3
1Department of Chemistry, Kalasalingam University,Krishnankoil – 626 190, India
2Department of Chemistry, Annamalai University, Annamalai nagar – 608 002, India
3Department of Chemistry, Ramco Institute of Technology, Rajapalayam – , India
DOI : http://dx.doi.org/10.13005/ojc/310269
Article Received on :
Article Accepted on :
Article Published : 04 May 2015
2:1 adducts involving [Zn(dtc)2]2 (dtc- = pipdtc-, H10C5NCS-2; dnpdtc-, (H3CCH2CH2)2NCS-2; dedtc-, (H5C2)2NCS-2; dmdtc-, (H3C)2NCS-2; nmedtc-, HOCH2CH2(CH)3NCS-2; deadtc-, (HOCH2CH2)2NCS-2) and 4,4' – bipyridine were prepared and characterized by elemental analyses, IR, UV-Visible, Cyclic voltammetric and thermal studies. IR spectra of the complexes show the reduction in the thioureide stretching frequency due to the increase in coordination around the zinc ion and the resultant increase in electron density. The charge transfer transitions are observed in the region 260 – 320 nm by electronic spectra. Thermal studies show a single stage weight loss. The cyclic voltammetric study on the complexes show an increase of electron density on zinc in the adducts compared to [Zn(dtc)2]2. The bond valence sum(BVS) analysis shows the values to be close to '2', which is equivalent to the formal oxidation state of zinc in the zinc complexes.
KEYWORDS:4;4’-bipyridine; dithiocarbamate; infrared spectra; thioureide; zinc
Download this article as:| Copy the following to cite this article: Manohar A, Ramalingam K, Karpagavel K. Synthesis, Characterization Studies and Bond Valence Sum (BVS) analysis on 2:1 Adducts involving Zinc(II)dithiocarbamates and 4,4’-Bipyridine. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Manohar A, Ramalingam K, Karpagavel K. Synthesis, Characterization Studies and Bond Valence Sum (BVS) analysis on 2:1 Adducts involving Zinc(II)dithiocarbamates and 4,4’-Bipyridine. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8782 |
Introduction
Zinc and cadmium dithiocarbamate complexes have continued to attract attention in recent years on account of their industrial applications1,2 and biological profiles.3,4 Dithiocarbamates are a family of compounds with diverse biological activities, suggesting that it may have multiple, clinical uses. Zinc and cadmium dithiocarbamates are used as single source precursor molecules to grow ZnS and CdS nanostructures respectively, for applications such as solar cells, photoconductors, lasers, optical waveguides, and non-linear integrated optical devices.5-7 Zinc dithiocarbamates and their adducts with primary and secondary amines have previously been prepared and some of these complexes are very active accelerators for the vulcanization of rubber by sulphur8 and the low temperature vulcanisation of latex.9
Tetrahedral complexes of divalent zinc and cadmium are known to expand their coordination shells by adding neutral nitrogenous ligand.10,11 The additional ligand shows its steric and electronic effects structurally on the complex formed. Synthesis, spectral, thermal, cyclicvoltammetry and single crystal X-ray structural studies on monomeric mixed ligand complexes involving bis(dithiocarbamato)zinc(II) and nitrogenous bases such as 2,2’-bipyridine, 1,10-phenanthroline, tetramethylethylenediamine (TMED),were reported extensively.12-18 All the adducts are containing discrete molecular units with ZnS4N2 chromophore in a distorted octahedral geometry. X-ray photoelectron spectroscopy (XPS), Cyclic voltammetry, C13 and H1NMR studies showed the increased electron density on metal centre on adduct formation. The crystal structures of dimeric zinc complexes involving zinc dithiocarbamates and 4,4’-bipyridine were reported from our laboratory.15,16 X-ray structural studies show that the zinc ion is five coordinated with a ZnS4N coordination environment in the dimeric complexes.
Since the crystal structures of some of the 4,4’-bipyridine adducts were reported earlier,15,19 in this paper, we report the results of spectral, thermal and electrochemical characterization studies of the adducts. In addition the results of the bond valence sum (BVS) calculations are also reported.
Experimental
Preparation of the Adducts
Preparation of [Zn2(dtc)4(4,4’–bipy)] (dtc–= pipdtc–, dnpdtc–, dedtc– )
The adducts were prepared by adding hot solutions of 4,4’–bipyridine (156mg, 1mmol) in chloroform to the hot solutions of respective parent [Zn(dtc)2]2 (2mmol) complex in chloroform. The resulting solution was left for evaporation at room temperature. Yellow precipitate of the adduct separated out and analysed to the proposed formula. [Zn2(pipdtc)4(4,4’–bipy)]: νmax = 1480 (C-N), 975 (C-S); (Found: C, 43.7; H, 5.0; N, 8.9%. Calc. for C34H48Zn2N6S8: C, 44.0; H, 5.2; N, 9.1 %); [Zn2(dnpdtc)4(4,4’–bipy)]: νmax = 1476 (C-N), 977 (C-S); (Found: C, 45.7; H, 6.3; N, 8.4%. Calc. for C38H64Zn2N6S8: C, 46.0; H, 6.5; N, 8.5 %); [Zn2(dedtc)4(4,4’–bipy)]: νmax = 1491 (C-N), 994 (C-S); (Found: C, 40.8; H, 5.4; N, 9.4%. Calc. for C30H48Zn2N6S8: C, 41.0; H, 5.5; N, 9.6 %);
Preparation of [Zn2(dtc)4(4,4′-bipy)](dtc–= nmedtc–, deadtc–).
The adducts were prepared by the methods reported earlier [15]. A hot ethanolic solution of [Zn(dtc)2]2 (430mg(deadtc–) or 325mg(nmedtc–), 2mmol) was added to a hot solution of 4,4’-bipyridine (156 mg, 1mmol) in ethanol. The resulting yellow solution was left for evaporation at room temperature. After two days yellow crystals separated out.
Preparation of [Zn2(dmdtc)4(4,4′-bipy)].
[Zn2(dmdtc)4(4,4′-bipy)] complex was prepared by the earlier method reported [12]. It was prepared by adding a hot solution of 4,4’-bipyridine (156mg, 1mmol) in benzene to a hot solution of [Zn(dmdtc)2]2 (2mmol) in benzene. The resulting yellow solution was left for evaporation at room temperature. After two days yellow precipitate of the adduct separated out and analysed to the proposed formula [Zn2(dmdtc)4(4,4′-bipy)].
Analytical and Physical Measurement
All the reagents and solvents employed were commercially available analytical grade materials, used as supplied, without further purification. IR spectra were recorded on a JASCO IR – 700 spectrophotometer (range 4000 – 400 cm-1) as KBr pellets. JASCO UVIDEC – 340 double beam spectrophotometer was used for recording the electronic spectra of the complexes. The spectra were recorded in alcohol, benzene and chloroform. BAS electrochemical analyzer was used for recording the cyclic voltammograms of the complexes. Working electrodes were hanging mercury drop electrode (HMDE) or platinum electrode, counter electrode was platinum wire and reference electrode was Ag/AgCl. The solvent, dichloromethane was purified by distillation methods. Supporting electrolyte was tetrabutylammonium perchlorate (0.1M). Experimental solution was thermostated at 25 ± 1°C and oxygen free atmosphere was provided by bubbling purified nitrogen through the solution.STA 1500 PL and Perkin – Elmer TGA7 Thermal Sciences instrument were used for the thermogravimetric analysis. The heating rate of the furnace was fixed at 10°C per minute. About 5mg of the sample was taken in porcelain crucible for each thermogravimetric experiment.
Results and Discussion
Infrared and Electronic Spectra
Important absorptions in the dithiocarbamate complexes are due to the nC–N and nC–S stretching modes. nC–N has been used as a measure of the contribution of the thioureide group to the structure of the dithiocarbamate. The polar nC = N+ appears at an intermediate value between the two extremes of (1250 – 1350 cm-1) and (1650 – 1690 cm-1).20,21 In this study nC–N for different [Zn(dtc)2]2 complexes appear in the region of 1485 – 1519 cm-1. In the case of adducts the nC–N values shift to lower wave number compared to the [Zn(dtc)2]2 complexes. The shift of nC–N to lower wave number in the case of adducts is due to the change in coordination geometry from tetrahedral to tetragonal pyramid.22 This observation is an indication of increase of electron density on zinc due to the adduct formation. The nC–S bands appear around 1000 cm-1 without splitting.23 The thioureide nC–N of the dithiocarbamates are well differentiated from those of 4,4′-bipyridine nC–N bands by comparison with their IR spectra.
The adducts are all faint to intense yellow colour. The electronic spectra of the adducts show transitions only due to charge transfer. The d–d transitions are absent as Zn(II) is d10 ion. The ligand transitions of the dithiocarbamate and the charge transfer transition in the adducts are observed in the region 260-320nm.
Cyclic Voltammetric Studies
The cyclicvoltammograms on the parent zinc dithiocarbamates and their 4,4’-bipyridine adducts were recorded in CH2Cl2 with TBAP as the supporting electrolyte. Cyclic voltammetric studies on simple [Zn(dtc)2]2 complexes at HMDE have been reported earlier.24 A two electron reduction process with the formation of zinc amalgam (Zn(Hg)) has been proposed.
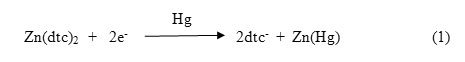
The CV responses for [Zn(nmedtc)2]2 _ 1.025 V corresponds to the process in Eq.(1). Oxidation of Zn(Hg) in the reverse scan was observed at _ 0.400 V. The process envisaged is:

The reduction potential at _ 1.500V is far higher in magnitude when compared to the parent [Zn(nmedtc)2]2 which is a two electron reduction process. 24,25 The observation
clear indication of the increased electron density on zinc in the adduct. The redox couple, _ 0.925/_0.675 was observed only when HMDE was used and was absent when a Pt electrode was used.
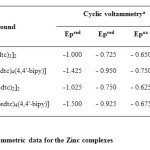 |
Table1: Voltammetric data for the Zinc complexes Click here to View table |
The involvement of an electroactive species produced as result of the interaction of mercury with the complex is envisaged.26,27 Nevertheless, the stoichiometry could not be established. Formation of a bimetallic complex involving dithiocarbamate is due to the inherent property of mercury to react with the complexes.28-30 The most important observation in the present study is the increased electron density on zinc during adduct formation. For other 4,4’-bipyridine adducts, also, a similar conclusion is arrived at in the present study. The cyclic voltammetry data are given in Table 1.
Thermogravimetric Analysis
The temperature range and percentage weight losses of the decomposition reactions are given in Table 2. The TG curves obtained for all the [Zn2(dtc)4 (4,4’-bipy)](where = dmdtc–,pipdtc– deadtc– , dedtc– and nmedtc–) adducts show a single stage weight loss, which ultimately leads to the formation of ZnS around 600°C. In the above mentioned complexes, the loss of 4,4’-bipyridine is not observed as an independent step. In contrast, a well defined step for the loss of 2,2’-bipyridine ligand is observed at 190°C in [Zn(dmdtc)2(2,2’-bipy]. But thermal decomposition of the dithiocarbamate moiety leads to the formation of ZnS completely around 600°C without any intermediate step for the formation of Zn(SCN)2. The above comparison shows that, the 4,4’-bipyridine adducts are thermally more stable than the 2,2’-analogues probably because of the dimeric nature. In addition, the TG analysis support the proposed molecular formulae of the complexes. Thermograms of [Zn2(dmdtc)4(4,4′-bipy)] and [Zn(dmdtc)2(2,2′-bipy)] are given in Fig.1. Dimeric structure of [Zn2(pipdtc)4(4,4′-bipy)] is given in Fig.2
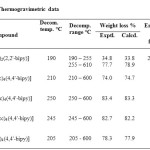 |
Table2: Thermogravimetric data Click here to View table |
![Fig 1. Thermograms of (a) [Zn2(dmdtc)4(4,4'-bipy)] and (b) [Zn(dmdtc)2(2,2'-bipy)]](http://www.orientjchem.org/wp-content/uploads/2015/05/Vol31_No2_Synth_ARUM_Fig1-150x150.jpg) |
Figure1: Thermograms of (a) [Zn2(dmdtc)4(4,4′-bipy)] and (b) [Zn(dmdtc)2(2,2′-bipy)]
|
![Fig 2. Dimeric structure of [Zn2(pipdtc)4(4,4'-bipy)]](http://www.orientjchem.org/wp-content/uploads/2015/05/Vol31_No2_Synth_ARUM_Fig2-150x150.jpg) |
Figure2: Dimeric structure of [Zn2(pipdtc)4(4,4′-bipy)] Click here to View figure |
Bond Valence Sum (BVS) Analysis
The bond valence sum method is popular method in coordination chemistry to estimate the oxidation states of atoms. The chemist wishing to estimate an unknown bond length in a molecule or crystal is confronted with intimidtating array of covalent radii, ionic radii and metallic radii etc., from which to choose.31 The bond valence method,32,33 has recently had considerable success in predicting and interpreting bond lengths in ‘ionic solids’. As it can be applied to estimate the bond lengths, vice-versa the sum of these bond lengths should give information about the valence of the central ion.
atoms i and j is defined so that the sum of all the bond valences from a given atom i with valence Vi obeys Σ vij = Vi
where:
vij = exp[(Rij – dij/B],
Rij = ri +r j – [rir j (Öci-Öcj)2] / [ ciri+cjrj]
and B is a universal constant equal to 0.37. The BVS values obtained for a number of dimeric complexes whose crystal structures have been reported from our laboratory and a few others reported from other laboratories, with two different sets of Rij values are presented in Table 3. Examination of the results clearly show the BVS values to be close to ‘2’, which is equivalent to the formal oxidation state of zinc in the complexes. Change in coordination number and change in coordination environment around the zinc ion have adjusted themselves in such a way that the valency of the central ion is satisfied.
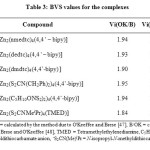 |
Table3: BVS values for the complexes Click here to View table |
Conclusion
Results of the analysis of the 4,4’-bipyridine adducts using IR, UV-Visible, CV, TG techniques and BVS analysis are presented in this paper. The thioureide band(νC-N) in the IR spectra of the complexes shifts to lower wave number. The observation is in line with the expected increase in electron density on the zinc ion. Electronic spectra of the adducts show weak bands in the region 260 – 320nm only due to charge transfer. The increased electron density on zinc in the adducts is well supported by the cyclic voltammetric studies. Thermal studies showed that the 4,4’-bipyridine adducts are thermally more stable than the 2,2’-analogues probably because of the dimeric nature. The BVS analysis has confirmed the valency of the central metal to be 2.0 as expected.
References
- Nieuwenhuizen J., Ehless A. W., Haashoot J. G.,, Janse S. R., Reedijk J., Baerends J., J. Am. Chem. Soc., 1999; 121: 163.
- McCleverty J. A., Gill S., J. Chem. Soc., Dalton Trans., 1982; 493.
- Casas J. S., Sanchez A., Bravo J., Garcia-Fontan S., Castellano E. E., Jones M. M., Inorg. Chim. Acta, 1989; 158: 119.
- Jones M. M., Jones S. G., Inorg. Chim. Acta, 1983; 79: 288.
- Das S. K., Morris G. C., J. Appl. Phys., 1993; 73: 782.
- Keitoku S., Ezumi E., Sol. Energy Mater. Sol. Cells, 1994; 35: 299.
- Seo K. W., Yoon S. H., Lee S. S., I.W. Shim I. W., Bull. Korean Chem. Soc., 2005; 26: 1582.
- Higgins G. M. C., Saville B., J. Chem. Soc., 1963; 2812.
- Mc Cleverty J. A., Gill S., J. Chem. Soc., Dalton Trans., 1982, 493.
- Klug H. P., Acta Crystallogr., 1996; 21: 536.
- Fraser K. A., Harding M. M., Acta Crystallogr., 1967; 22: 75.
- Manohar A., Venkatachalam V., Thirumaran S., Ramalingam K., Bocelli G., Cantoni A., J. Chem. Crystallogr., 1998; 28: 86.
- Thirumaran S., Ramalingam K., Bocelli G., Cantoni A., Polyhedron, 1999; 18: 925.
- Ramalingam K., Uma S., Rizzoli C., Marimuthu G., J. Coord. Chem., 2010; 63: 4123.
- Manohar A., Ramalingam K., Bocelli G., Righi L., Inorg. Chim. Acta, 2001; 314: 177.
- Marimuthu G., Ramalingam K., Rizzoli C., Polyhedron, 2010; 29: 1555.
- Srinivasan N., Thirumaran S., Ciattini S., J Mol. Struct., 2009; 936: 234.
- Arul Prakasam B., Ramalingam K., Bocelli G., Cantoni A., Polyhedron, 2007; 26: 4489.
- Lai C. S., Tiekink E. R. T., Appl. Organometal. Chem., 2003; 17: 253.
- R. Baggio, A. Frigerio, E.B. Halac, D. Vega and M. Perec, J. Chem. Soc. Dalton Trans., 1992, 549.
- Aravamudan G., Brown D. H., Venkappayya D., J. Chem. Soc.(A), 1971; 2744.
- Hassaan A. M. A., Soliman E. M., El-Roudi A. M., Polyhedron, 1989; 8: 925.
- Bonati F., Ugo R., J. Organometal. Chem., 1967; 10: 257.
- Bond A. M., Hollen-Kamp A. F, Inorg. Chem., 1990; 29: 284.
- Budniknov G. K., Ulakhovich N. A., Zh. Obshch. Khim., 1976; 46: 1129.
- Aggarwal R. C., Singh B., Singh M. K., J. Indian Chem. Soc., 1982; 59: 269.
- Aggarwal R. C., Singh B., Singh M. K., Indian J. Chem., 1993; 22: 533.
- Bond A.M., Thackery J. R., Casey A. T., Inorg. Chem., 1973; 12: 887.
- Joris S. J., Aspila K. I., Chakraparti C. L., Anal. Chem., 1969; 41: 1441.
- Randle T. H., Cardwell T. J., Magee R. J., Aust. J. Chem., 1975; 28: 21.
- Pauling L., The Nature of Chemical Bonding, 3rd edn., Cornell Ithaca, New York, USA, 1960.
- Brown I.D., Structure and Bonding in Crystals, M. O’ Keeffe A. Navrotsky, Eds., Vol. 2 Academic, New York, 1981.
- Keeffe M. O., Structure and Bonding, 1989; 71: 162.
- Summers S. P., Abbound K. A., Farrah S. R., Palenik G. J., Inorg. Chem., 1994; 33: 88.
- Keeffe M. O., Brese N. E., J. Am. Chem. Soc., 1991; 113: 3226.
- Brese N. E., Keeffe M. O., Acta Crystallogr. Sec.B, 1991; 47: 192.
- Ray N. K., Samuels L., Parr N. G., J. Chem. Phys. 1979; 70: 3680.
- Brown I.D., Altermatt D., Acta Crystallogr., 1985; B41: 244.

This work is licensed under a Creative Commons Attribution 4.0 International License.









