Simulation Studies of the Damaging Effect of Molecular Hydrogen gas on Double Walled Carbon Nanotubes.
Ali Ershadi*
Department of Chemistry, science and Research Branch, Islamic Azad University, Tehran, Iran. majid_kuala@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310270
Article Received on :
Article Accepted on :
Article Published : 08 Jun 2015
The hydrogen damage in the double walled carbon nanotubes (DWCNTs) was studied through molecular simulation.The simulation performed in the measuring domain has the dimensions of about 20×20×13 A. Our calculations revealed that the partial pressure of the hydrogen gas between the two walls is 180 atm and this pressure is equal to hydrogen density of 7.6 mol/L.This simulation procedure showed that the partial amount of the hydrogen molecules gas at first dissociated and then penetrate to the external wall of DWCNT.This approach is similar to hydrogen embrittlement on metals surface.
KEYWORDS:Simulation; Studies; Damaging Effect; Molecular Hydrogen
Download this article as:| Copy the following to cite this article: Ershadi A. Simulation Studies of the Damaging Effect of Molecular Hydrogen gas on Double Walled Carbon Nanotubes. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ershadi A. Simulation Studies of the Damaging Effect of Molecular Hydrogen gas on Double Walled Carbon Nanotubes. Available from: http://www.orientjchem.org/?p=9126 |
Introduction
Recently different storage technologies have been explored to develop a secure and cheap method to save hydrogen[1,2].Undoubtedly, the surface texture of carbonaceous materials is one of the most important factors for hydrogen storage.Pores with two or three times the diameter of a hydrogen molecule were illustrated as the optimum dimension for hydrogen storage, because high gas density was acquired in the fine pores from the calculated results[3-7].
Carbon nanotubes are novel carbons which attracted great interest among the scientific society owing to a wide range of characteristics including electronic, mechanical and other properties[8-13].
Nowadays, there has been a good deal of experimental and theoretical interest in the possibility of hydrogen storage in single walled carbon nanotubes (SWNTs), multi-walled carbon nanotubes(MWNTs) as well as carbon nanofibers[14-21].
It is usually approved that the majority of hydrogen adsorbed in SWNTs was physical adsorption on their inside surface, as well as in the interstitial sites between nanotube bundles[22-28].
It has been found that hydrogen must initially find uncovered graphene edges where it dissociates into atomic hydrogen. These sites would then prepare path ways for the atomic hydrogen to migrate inward to occupy the space between the basic planes [29-35].
This event resembles hydrogen damage in metal structures.Hydrogen damage is a general term which includes:Hydrogen Blistering and Hydrogen Embrittlement.Hydrogen blistering and embrittlement are results of the hydrogen penetration into a metal.The consequence is local distortion and in extremecases complete destruction of the vessel wall[36-41].
In the past several years various experimental [42,43]and theoretical [44,45]works have been devoted to hydrogen adsorption on carbon nanotubes [46-48].
Considerable researches have been performed about carbon nanotube due to its versatile applications in the fields such as drug delivery [49,50], super capacitors [51-53],gas adsorption [54-56] chemical [57-59] and electrochemical [60-62] sensors. There are several magnificent books [63-65] and reviews [66-68] that describe synthesis, processing, characterization and applications of carbon nanotubes [69-71].
In the present work the structural damage of double walled carbon nanotubes when treated with high pressure molecular hydrogen gas have been investigated by simulation approach [72-74].
Molecular Gasadsorption Simulation
The physisorption of hydrogen was studied in an array fragment including 48 carbons. The investigation was carried out on double walled carbon nanotube with chirality (10, 0),Length 13 A and a=5A, b=10A (a and b are inner and outer radius of cylinder, respectively), Fig.1.
It was assumed that C-H bonds were located on the terminal of carbon nanotube.ThreeH2molecules were supposed to be located between two walls of carbon nanotubes. The calculated pressure of hydrogen molecules is about 180atm which is corresponding to hydrogen gas density of about 7.6 mol/L.
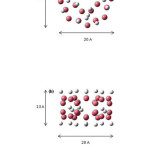 |
Figure1: First system configuration used for optimization by the Gaussian09 package, (a) top view and (b) side view. Arrows indicate the dimension of the simulated system. Click here to View figure |
The hydrogen adsorption in double walled carbon nanotubes arrays was investigated by molecular simulation.The double walled carbon nanotube structure was simulated by Nanotube Modeler3 package and then simulation was completed by Gausian0 9 computational package [75-79].
The calculations showed thatat P=180atm and T=300K two hydrogen molecules were adsorbed by external wall of double walled carbon nanotube and one hydrogen molecule has been dissociated as shown Fig.2(b) and the two resulted hydrogen atoms deformed the external wall of the double walled carbon nanotube as indicated in Fig.2(d).Results of the above-mentioned simulation are illustrated in table 1.
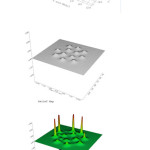 |
Figure2: Several system configurationsof electron density Click here to View figure |
In the measuring domain of dimensions 20×20×13 A, we placed an array of 76 atoms,(48 carbon atoms plus 24 hydrogen atoms)and 3 hydrogen molecules,Fig 1.
Table1: The calculation summary of simulation and optimization procedure.
|
Calculation type |
Basis set |
E(RHF) (a.u.) |
RMS Gradient Norm (a.u.) |
|
FOPT |
B3LYP |
-1834.3 |
0.057 |
The simulation was carried out until optimization was approached,after which from the molecular properties, the macro parameters were determined.Within the framework of this study partial dissociation of hydrogen molecules and penetration of hydrogen atoms into external wall of double walled carbon nanotube was assumed to be similar to embrittlement phenomenon in metals [80-82].
Results and Discussion
Atomic hydrogen (H) is the only species capable of penetrating into metals and alloys.Molecular hydrogen (H2) does not penetrate into metals and alloys[36].It can be assumed that similar process is occurred during penetration of hydrogen into the surface of DWCNTs [83-85].
Simulation procedure showed final location of hydrogen molecules related to the DWCNT which yielded most stable state of the system with minimum energy of approximately-1834.3 hartree, Fig.3.
The simulation results showed that at 300K inside of the double walled carbon nanotube, two factors are critical for hydrogen adsorption:
- Distance of the hydrogen gas molecules from the walls of nanotube before penetration
- Partial dissociation of hydrogen molecules and embrittlement in carbonnanotube surface.
The pressure of hydrogen gas molecules between the walls of DWCNT was calculated to be about 180atm; but according Heisenberg uncertainty principle, in this space, measurement of pressure is limited by equation of the type:
 |
Figure3: Total energy (a) and RMS gradient (b)change during optimization step. |
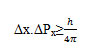
Where Δx is the uncertainty in the distance between the walls of DWCNT which is equal to 5A andΔPx is the uncertainty in a particular component of momentum that calculated according to the following equation:
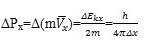
Where m is mass of hydrogen molecule (m=3×10-24), so that uncertainty in the kinetic energy of molecular hydrogen is given by:
![]()
According to the perfect gas model, the internal energy of the gas is equal to its kinetic energy, therefore:
![]()
Since the Equation of State for perfect gas is, PVm=NAKT therefore the uncertainty in kinetic energy of the gas is:
![]()
Where Vm=0.13 L. mol-1 and NA=6.02×1023. Therefore ΔP=6.5×1013 atm. This huge uncertainty shows that microscopic and macroscopic pressures are very different.
Conclusion
Using the molecular optimizing simulation method, the pressure of the molecular hydrogen gas between the walls of DWCNT and average distance between hydrogen species and the surface of the DWCNT has been calculated.
The specific location of hydrogen molecules with respect to hexagonal rings of DWCNT wall is an indication of hydrogen penetration into carbon nanotube walls. In the previous works these centers referred to as “carbon islands”[86].
The calculated results reveal that hydrogen molecules are physisorbed on the center of carbon nanotube hexagonal ring. Exposed carbon edges as “carbon islands” have a significant contribution in hydrogen adsorption. Hydrogen adsorbed in the DWCNTs could not be entirely released from the surface of the carbon nanotube [87-89].
Hydrogen molecules dissociate inside of the center of hexagon and are chemisorbed by carbon atoms. This process causes hydrogen damage of carbon nanotube. A fraction of the pore structure of the DWCNTs was permanently destructed by hydrogen adsorption.
Based on the performed simulation, it seems that in physisorb and Chemisorb of hydrogen on double walled carbon nanotubes, internal wall is unperturbed but external wall is damaged by molecular and atomic hydrogen. This research proposed that the amount of adsorbed hydrogen is proportional to the outer diameter of DWNTs. These results are consistent with the previously reported works [44,45,90]although a complete study of this aspect is required.
References
- Lee, SY.; Rhee, KY.; Nahm, SH .; Park, SJ.J Solid State Chem.2014, 210, 256–60
- Silambarasan, D.; Vasu, V.; Iyakutti, K.; Surya, VJ.Physica E.2014, 60, 75–9
- Chen, CH.; Huang, CC.Sep Purif Technol.2009,65, 305–10
- Monajjemi, M.;Baei, M.T.;Mollaamin, F.Russian Journal of Inorganic Chemistry.2008, 53 (9), 1430-1437
- Monajjemi,M.; Rajaeian,E.; Mollaamin,F.; Naderi,F.; Saki,S.Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi,M.;Seyed Hosseini,M.Journal of Computational and Theoretical Nanoscience.2013,10 (10), 2473-2477
- Yahyaei,H.;Monajjemi, M.Fullerenes, Nanotubes, and Carbon Nano structures. 2014,22(4), 346–361
- Zhang, L.;Castillejos, E.;Serp, Ph.; Sun, WH.; Durand, J.Catal Today2014.
- Machado, BF.;Oubenali, M.;Axet, MR.; Nguyen, TT.;Tunckol, M.;Girleanu,M.;Ersen, O.; Gerber, IC.Serp,Ph,J Catal2014, 309, 185–98
- Monajjemi, M.; Jafari Azan, M.;Mollaamin,F.Fullerenes, Nanotubes, and Carbon Nanostructures.2013,21(6), 503–515
- Nafisi, S.;Monajemi, M.;Ebrahimi, S. Journal of Molecular Structure. 2004,705 (1-3)35-39
- Monajjemi, M.;Baheri,H.;Mollaamin,F. Journal of Structural Chemistry.2011 52(1), 54-59
- Monajjemi, M.; Seyed Hosseini,M.;Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Dundar-Tekkaya E.; Karatepe N. Int J Hydrogen Energy.2014,10.145
- Wenelska, K.;Michalkiewicz, B.; Chen, X.;Mijowska, E. Energy2014,75,549-54
- Mahdizadeh,SJ.;Goharshadi, EK.;Int j hydrogen energy.2014,39, 1719-31
- Monajjemi, M.; Boggs, J.E.J. Phys. Chem. A,2013,117,1670 −1684
- Monajjemi ,M.; Honaparvar ,B.; KhaliliHadad ,B.; Ilkhani ,AR.; Mollaamin,F. Afr. J. Pharm. Pharmacol .2010, 4 (8), 521-529
- Monajjemi, M.Chemical Physics. 2013, 425, 29-45
- Fazaeli ,R.; Monajjemi ,M.; Ataherian ,F.; Zare,K.Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi,M.;Mollaamin,F,J ClustSci, 22(2011)673.
- Seenithurai, S.;KodiPandyan, R.;Vinodh Kumar, S.;Mahendran,M. Int j hydrogen energy, 2013, 38,7376-81
- Kanmani, M.;Lavanya, R.;Silambarasan, D.;Iyakutti, K.; Vasu, V.; Kawazoe, Y. Solid State Commun,2014,183, 1–7
- Lu, J.; Xiao ,H.; Cao, J. J Solid State Chem,2012, 196, 367–71
- Monajjemi,M .; Aghaie , H.; Naderi ,F. Biochemistry (Moscow).2007,72 (6), 652-657
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Monajjemi ,M.; Chahkandi ,B.; Zare,K.; Amiri, A. Biochemistry (Moscow),200570 (3), 366-376
- Monajjemi, M .; Afsharnezhad,S.; Jaafari, M.R.; Abdolahi,T.; Nikosade ,A.;Monajemi ,H.; Russian Journal of physical chemistry A, 2007,2,1956-1963
- Hou, PX.; Xu, ST.; Ying, Z.; Yang, QH.; Liu , C.; Cheng HM. Carbon. 2003,41,2471–76
- Soldano, GJ.; Juarez ,MF.;Teo, BWT.; Santos, E. Carbon 2014,78, 181 –9
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4), 673-692
- Mollaamin ,F.;Monajjemi , M , Journal of Computational and Theoretical Nano science.2012, 9 (4) 597-601
- Monajjemi,M.Struct. Chem,2012, 23551.
- Mollaamin,F.; Gharibe, S.; Monajjemi,M. Int. J. Phy. Sci , 2011,6, 1496-1500
- Monajjemi, M .; Faham, R.;Mollaamin, F.Fullerenes, Nanotubes, and Carbon Nanostructures,201220, 163–169
- Revie,RW.;editor. Uhlig’s corrosion handbook. 4th Ed. New York: John Wiley & Sons; 2011; p. 183-4.
- Monajjemi,M.; Khaleghian,M.; Tadayonpour,N.; Mollaamin,F. International Journal of Nanoscience,2010,9 (05), 517-529
- Mollaamin , F.;Najafi ,F.;Khaleghian,M.;KhaliliHadad,B.;Monajjemi ,M. Fullerenes, Nanotubes, and Carbon Nanostructures,201119, 653–667
- Monajjemi, M.; Chegini , H. ; Mollaamin , F. ; Farahani ,P,Fullerenes, Nanotubes, and Carbon Nanostructures.2011,19, 469–482
- Monajjemi ,M.; Yamola,H.;Mollaamin,F.Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Monajjemi, M.; Heshmata, M.; Haeri, HH , Biochemistry (Moscow),2006, 71 (1), S113-S122
- Barghi, SH.;Tsotsis, TT.;Sahimi, M. int j hydrogen energy2014,39, 1390-97
- Tylianakis, E.;Dimitrakakis, GK.; Martin-Martinez, FJ.; Melchor, S.;Dobado, JA.; Klontzas, E.; Froudakis, GE. Int J Hydrogen Energy2014.
- Kovalev ,V.;Yakunchikov, A, Acta Astronaut2011,68, 681–85
- Ng, TY.; Ren, YX.;Liew, KM. int j hydrogen energy,2010,35,4543–53
- Monajjemi, M.;Falahati,M.;Mollaamin,F.;Ionics,2013, 19, 155–164
- Monajjemi , M.; Heshmat ,M.; Aghaei , H.; Ahmadi , R.; Zare,K.Bulletin of the Chemical Society of Ethiopia, 2007,21 (1)
- Monajjemi,M.; Lee, V.S. ;Khaleghian,M.; B. Honarparvar, B.; F. Mollaamin, F,J. Phys.Chem. C. 2010,114 (2010) 15315
- Wong, BS.;Yoong, SL.;Jagusiak,A.;Panczyk,T.; Ho, HK.;Ang, WH.;Pastorin, G. Adv Drug Deliver Rev. 2013; doi:10.1016/j.addr.2013.08.005.
- Wu, H.; Shi, H.; Zhang,H.;Wang,X.; Yang,Y.;Yu,C.;Hao, C.; Du, J.; Hua, H.; Yang, S. Biomaterials 2014; 35: 5369-80.
- Wang, B.;Qiu, J. Feng H, Sakai E, Preparation of graphene oxide/polypyrrole/multi-walled carbon nanotube composite and its application in supercapacitors, Electrochim Acta.
- Fedorovskay EO, B; ulusheva, L;.Kurenya, AG.; Asanov, IP.;Rudina, NA.; Funtov, KO.;Lyubutin, IS.;Okotrub, AV. Electrochim Acta2014,139,165–72
- Yang,H.;Wang, N.; Ren ,Y.;Cai,L.;Chen,Z.; Xu ,Q. Mater Lett 2014.
- Yu, JG.; Yu LY ,Yang,.; H, Liu ,Q.; Chen XH, Jiang ,X.; Chen, XQ.; Jiao, FP. Scie Total Environ 2015,502,70–9
- Liu, L.; Nicholson, D.; Bhatia, SK.;ChemEngSci2014
- Lei, G.; Liu, C.;Xie, H.; Liu ,J Chem Phys Lett,2014, 232–6, 616–617
- Mittal M, Kumar A. Sens Actuator B 2014.
- Nur Kaya, E.;Tuncel,S.;Basova, TV.;Banimuslem,H.;Hassan,A.;Gürek,AG.;Ahsen, V.; Durmus, M. Sens Actuator B2014,199, 277–83
- Jin, HJ.; An JM, Park,J.;Moon,SJ.;Hong, S. BiosensBioelectron,2013,49, 86–91
- Zhang, H.; Zhang, J.; Zheng, J. Measurement 2015,59,177–83
- Lorestani, F.;Shahnavaz, Z.;Mn, P.; Alias, Y.; Manan, NSA.. Sens Actuator B 2015; 208: 389–98.
- Barsan MM, Toledo CT, Brett CMA. New electrode architectures based on poly(methylene green) and functionalized carbon nanotubes: characterization and application to detection of acetaminophen and pyridoxine, J ElectroanalChem 2014.
- Terranova,ML.;Orlanducci,S.; Rossi,M.;editors. Carbon Nanomaterials for gas Adsorption. Boca Raton: Taylor & Francis; 2012.
- TagmatarchisN.;editor. Advances in carbon nanomaterials science and applications. Boca Raton: Taylor & Francis; 2012.
- Monthioux M.; editor. Carbon Meta-Nanotubs: Synthesis, Properties and Applications. Chichester: John Wiley&Sons; 2012.
- Herrero-Latorre C.; Álvarez-Méndez J.;Barciela-García J.; García-Martín S.; Peña-Crecente RM. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal Chim Acta 2014.
- Herrera-Herrera, AV.; González-Curbelo MÁ.; Hernández-Borges J.;Rodríguez-Delgado MÁ. A review. Anal ChimActa2012, 734, 1– 30.
- Amin, AM.;Croiset, E.;Epling, W. int j hydrogen energy2011; 36, 2904-35
- Monajjemi, M.;Sobhanmanesh, A.;Mollaamin,F.Fullerenes, Nanotubes, and Carbon Nanostructures,2013,21 47–63
- Ariafard,A.; Fazaeli,R.; Aghabozorg,HR.; Monajjemi, M. Journal of Molecular Structure: THEOCHEM,2003,625 (1), 305-314
- Monajjemi ,M.; Karachi ,N.;Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures, ,2014, 22: 643–662
- Monajjemi, M.; Mahdavian,L.; Mollaamin,F.: Bull .Chem.Soc.Ethiop ,2008,22(2),1-10.
- Mollaamin ,F.; Baei, MT.; Monajjemi, M.; Zhiani , R.;Honarparvar , B.; Russian Journal of Physical Chemistry A, Focus on Chemistry,2008, 82 (13), 2354-2361
- Monajjemi, M.; Ghiasi, R. ;Ketabi, S. Journal of Chemical Research.2004, 1: 11-18
- Mahdavian,L.; Monajjemi, M.; Mangkorntong ,N. Fullerenes, Nanotubes and Carbon Nanostructures,2009, 17 (5), 484-495
- Monajjemi, M .; Ketabi ,S.; Amiri, A. Russian Journal of Physical Chemistry , 2006, 80 (1), S55-S62
- M. Monajjemi .; Robert Wayne Jr, J.E. Boggs, Chemical. Physics. 433 (2014) 1-11
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society,2006, 50 (4), 143-148
- Monajjemi ,M.; Mollaamin ,F.Journal of Computational and Theoretical Nanoscience,2012 ,9 (12) 2208-2214
- Monajjemi, M.; Mahdavian ,L . ; Mollaamin, F.; Honarparvar, B.Fullerenes, Nanotubes and Carbon Nanostructures,201018, 45–55
- Ghalandari,B.;Monajjemi,M.;Mollaamin,F.;Journal of Computational and Theoretical Nanoscience,20118, 1212–1219
- Monajjemi,M.;Khosravi,M.; Honarparvar,B.;Mollaamin,F.;International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Monajjemi,M.; Rajaeian, E.; Mollaamin,F. Physics and Chemistry of Liquids,2008,46 299.
- Tahan, A.; Monajjemi,M. Acta Biotheor,2011, 59, 291–312
- Monajjemi ,M.;Farahani,N.;Mollaamin ,F. Physics and Chemistry of Liquids,2012 , 50( 2) 161–172
- Zhu ,W.;Börjesson,A.;Bolton, K.; Carbon 2010, 48, 470–78
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.;Russian Journal of Physical Chemistry A, 2008 , 82 (13), 2277-2285
- Mollaamin , F.; Varmaghani , Z.; Monajjemi ,M, Physics and Chemistry of Liquids.2011,49318
- Monajjemi,M.; Honarparvar, B.; H. Haeri, H.; Heshmat, M.; Russian Journal of Physical Chemistry C, 2008, 80(1):S40-S44.
- Hou ,PX.; Xu, ST.; Ying, Z.; Yang QH, Liu C.;Carbon 2003, 41, 2471–76

This work is licensed under a Creative Commons Attribution 4.0 International License.









