Preparation of Arylamines form Aldehydes by Zn(BH4)2/MgBr2
Mina Mahmoudi and Davood Setamdideh*
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135-443, Iran. Corresponding Author Emails : davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310280
Article Received on :
Article Accepted on :
Article Published : 19 Jun 2015
Reductive aminationa variety of aldehydes with different anilines has been performed by Zn(BH4)2/MgBr2 as reducing system in THF at room temperature in high to excellent yields of the corresponding secondary amines (80-90%).
KEYWORDS:Zn(BH4)2; MgBr2; Reductive amination; Aldehydes
Download this article as:| Copy the following to cite this article: Mahmoudi M, Setamdideh D. Preparation of Arylamines form Aldehydes by Zn(BH4)2/MgBr2. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Mahmoudi M, Setamdideh D. Preparation of Arylamines form Aldehydes by Zn(BH4)2/MgBr2. Available from: http://www.orientjchem.org/?p=9331 |
Introduction
One’s famous of pharmaceutical intermediates for the synthesis of some drugs are amines. These compounds can be obtained by the reduction of cyano, azide, nitro, carboxamide derivatives. On the other hands, direct reductive amination (DRA) is a single operation can be carried out by some non-borohydride reducing system that they have been reviewed by our former works; also some borohydrides systems have been used for DRA reaction 1.These methods have advantages and some disadvantages such as long reaction time, excess amount of reagents, using expensive reagents, toxic byproducts and higher reaction temperature. Therefore, there is interest in synthesis of amines under new systems. Recently, we have reported that NaBH4/DOWEX(R)50WX41a, NaBH4/B(OH)3& Al(OH)31b, NaBH4/Ga(OH)31c, NaBH4/C 1d and NaBH4/NaH2PO4.H2O1e can be used as convenient systems for reductive amination of aldehydes.
So, in continuing our efforts for the development of new reducing systems1-2,we have re-examined the DRA reaction.Thus, we introduce an efficient systemi.e. Zn(BH4)2/MgBr2 in THF at room temperature for reductive amination of aldehydes.
Results and Discussion
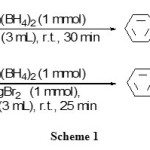
The model reaction has been chosen by reductive amination of benzaldehyde with aniline. This reaction was performed in different conditions as shown in table 1. By the testing of the different solvents, we have found THF is better solvent because it gives the highest yield of product. The optimization reaction conditions showed that using 1 molar equivalents of Zn(BH4)2 and 1 molar equivalents of MgBr2 in THF were the best conditions to complete the reductive amination of benzaldehye (1 mmol) and aniline (1 mmol) to N-benzylaniline (Table 1, Entry4). The reaction was completed within 25 min with 90% yields of product as shown in scheme 1.
 |
Scheme 1 Click here to View scheme |
Table1: Optimization of the Reductive Amination of Benzaldehyde (1 mmol) and Aniline (1 mmol) to Benzylaniline with Zn(BH4)2 and MgBr2 at Room Temperature.
| Table 1. Optimization of the Reductive Amination of Benzaldehyde (1 mmol) and Aniline (1 mmol) to Benzylaniline with Zn(BH4)2 and MgBr2at Room Temperature. | |||||
|
Entry |
Zn(BH4)2/mmol |
MgBr2/mmol |
Solvent/3 mL |
Time/min |
Yieldsa /% |
|
1 |
0.5 |
1 |
THF |
60 |
50 |
|
2 |
0.5 |
0.5 |
THF |
60 |
30 |
|
3 |
1 |
0.5 |
THF |
60 |
50 |
|
4 |
1 |
1 |
THF |
25 |
90 |
|
5 |
1 |
1.5 |
THF |
20 |
85 |
|
6 |
1.5 |
1 |
THF |
25 |
50 |
|
7 |
1 |
1 |
CH3CN |
60 |
50 |
|
8 |
1 |
1 |
Et2O |
60 |
50 |
|
9 |
1 |
1 |
CH2Cl2 |
60 |
20 |
aYields refer to isolated pure benzylaniline.
The efficiency of this protocol was further examined by using various structurally different aldehydes and anilines. In this approach, the corresponding secondary amines were obtained in excellent yields (80-90%) and within appropriate times (20-45 min) as shown in Table 2.
Table2-1: Reductive Amination of Aldehydes (1 mmol) with Anlines (1 mmol) by Zn(BH4)2 (1 mmol) in the presence of MgBr2 (1 mmol) in THF (3 mL) at Room Temperature.
|
Table 2-1. Reductive Amination of Aldehydes (1 mmol) with Anlines (1 mmol) by Zn(BH4)2 (1 mmol) in the presence of MgBr2 (1 mmol) in THF (3 mL) at Room Temperature. |
||||||
|
Entry |
Aldehydes |
Anilines |
Products |
Time/ min |
Yields a/ % |
|
|
1 |
benzaldehyde |
aniline |
N-benzylaniline |
25 |
90 |
|
|
2 |
benzaldehyde |
4-bromoaniline |
N-benzyl-4-bromoaniline |
20 |
87 |
|
|
3 |
benzaldehyde |
4-methoxyaniline |
N-benzyl-4-methoxyaniline |
20 |
90 |
|
|
4 |
2-nitrobenzaldehyde |
4-methylaniline |
N-(2-nitrobenzyl)- 4-methylaniline |
20 |
89 |
|
|
5 |
4-bromobenzaldehyde |
aniline |
N-(4-bromobenzyl)aniline |
20 |
82 |
|
|
6 |
2-methoxybenzaldehyde |
aniline |
N-(2-methoxybenzyl)aniline |
30 |
89 |
|
|
7 |
4-methylbenzaldehyde |
4-bromoaniline |
N-(4-methylbenzyl)-4-bromoaniline |
30 |
90 |
|
|
8 |
2-nitrobenzaldehyde |
4-methoxyaniline |
N-(2-nitrobenzyl)- 4-methoxyaniline |
20 |
90 |
|
|
9 |
4-methylbenzaldehyde |
4-methylaniline |
N-(4-methylbenzyl)- 4-methylaniline |
20 |
83 |
|
|
10 |
4-methylbenzaldehyde |
4-methoxyaniline |
N-(4-methylbenzyl)- 4-methoxyaniline |
40 |
89 |
|
|
11 |
4-methoxybenzaldehyde |
4-methylaniline |
N-(4-methoxybenzyl)-4-methylaniline |
20 |
90 |
|
|
12 |
2-methoxybenzaldehyde |
4-bromoaniline |
N-(2-methoxybenzyl)-4-bromoaniline |
45 |
89 |
|
|
13 |
4-nitrobenzaldehyde |
aniline |
N-(4-nitrobenzyl)aniline |
20 |
85 |
|
|
14 |
cinnamaldehyde |
aniline |
N-cinnamylaniline |
20 |
80 |
|
a Yields referee to isolated pure products.
The rate-determining step for reductive aminations is Imine formation therefore additions of co-reactants are desirable. It is notable, in the absence of MgBr2, imine does not generate and benzyl alcohol has been produced as shown in scheme 1.
Also, the reductive amination of cinnamaldehyde with 1 molar equivalents of aniline and aniline by 1 molar equivalents of Zn(BH4)2 and 1 molar equivalents of MgBr2 was carried out exclusively in 1,2-reduction manner within 20 minutes at room temperature. In these reactions the corresponding cinnamylanilines were obtained in 80% yields (Table 2, entry 14).
The products were determined from the 1H-chemical shift of the CH2 group which appeared around 4.22-4.68 ppm as a singlet. Also the NH stretching frequency in FT-IR spectrum appeared around 3380-3427 cm-1.
Experimental
General
All substrates and reagents were purchased from commercially sources with the best quality and used without further purification. IR and 1H NMR spectra were recorded on Perkin Elmer FT-IR RXI, 100 and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Reductive amination of benzaldehyde and aniline with Zn(BH4)2/MgBr2, A typical procedure
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a solution of benzaldehyde (0.106 g, 1 mmol) , aniline (0.093 g, 1 mmol) and MgBr2(0.182 g, 1 mmol) in THF (3 mL) was prepared. The resulting mixture was stirred for 5 min at room temperature. Then Zn(BH4)2(0.275 g, 1 mmol) was added to the reaction mixture and stirred at room temperature. TLC monitored the progress of the reaction (eluent; CCl4/Ether: 5/2). The reaction was filtered after completion within 25 min. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Ether: 5/2) afforded the N-benzylaniline (0.l64 g, 90% yield, Table 2, entry 1).
Conclusions
In this context, we have shown that Zn(BH4)2/MgBr2 is suitable for the reductive amination of a variety of aldehydes and anilines to their corresponding secondary amines in high to excellent yields. Reduction reactions were carried out with 1 molar equivalents of Zn(BH4)2 in the presence of 1 molar equivalents of MgBr2 in THF at room temperature. High efficiency of the reductions, shorter reaction times and easy work-up procedure makes as an attractive new protocol for reductive amination of aldehydes.
Acknowledgments
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
- a) Setamdideh, D.; Sepehraddin . D. J. Mex. Chem. Soc. 2014, 57, 22-25. b) Setamdideh, D.; Hasani, S.; Noori, S. J. Chin. Chem. Soc.2013, 60, 1267-1271. c) Pourhanafi, S.; Setamdideh, D.;Khezri, B. Orient. J. Chem. 2013, 29, 709-712.d) HsanloieTaie, S.; Setamdideh, D. Orient. J. Chem. 2014, 30, 341-344. e) S.; Arefi, H.; Setamdideh, D. Orient. J. Chem. 2014, 30, 299-302.
- a) Setamdideh, D.; Rafigh, M. E-J. Chem. 2012, 4, 2338-2345. b) Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc. 2012, 56, 169-175.c) Setamdideh, D.; Ghahremani, S. S. Afr. J. Chem.2012, 65, 91-97. d) Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc.2013, 78, 1-13e) Mohamadi, M.; Setamdideh, D.; Khezri, B. Org. Chem. Inter.2013, 2, doi:10.1155/2013/127585.f) Setamdideh, D.; Khaledi, L. S. Afr. J. Chem.2013, 66, 150-157.g) Azizi Asl, P.; Setamdideh, D, J. Chin. Chem. Soc.2014, 61, 940-944.

This work is licensed under a Creative Commons Attribution 4.0 International License.









