One-Pot Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives
Seyyedeh Naghmeh Sadat, Farhad Hatamjafari*
Department of Chemistry, College of Science, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/310275
Article Received on :
Article Accepted on :
Article Published : 21 Apr 2015
An efficient, simple and one-pot protocol for synthesis of 1,8-Dioxo-octahydroxanthene derivatives via multi-component reactions between dimedone and various aromatic aldehydes employing barium perchlorate as catalyst is described. The structural features of the synthesized compounds were characterized by IR and 1H NMR. The presented method is available, environmentally friendly, cheap and highly effective to give the products in good to excellent yields.
KEYWORDS:Xanthenes; Solvent-free; Multicomponent reactions; One-pot.
Download this article as:| Copy the following to cite this article: Sadat S. N, Hatamjafari F. One-Pot Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Sadat S. N, Hatamjafari F. One-Pot Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8547 |
Introduction
In recent years, the uses of catalysts supported on solid supports have been extensively developed because such catalysts not only cause to simplify the purification processes but also do not release toxic substances residues into the environment. Although some of them are sensitive to moisture and can be easily decomposed, application of them in organic reactions is difficult. This problem can be solved by fixed onto solid supports1-3.
Xanthene derivatives are one of the important classes of organic compounds and there are many applications which are biologically important drug intermediates in the field of medicinal chemistry for their biologically active properties, such as antinociceptive activities as well as their efficiency in photodynamic therapy antimalarial, antibacterial, antiinflammatory, and antiviral properties and have been used as dyes, fluorescent material and in laser technologies6-12. Recently, several improved methodologies have been developed that use HClO4–SiO2 13, ZnO 18 triethylbenzyl phosphomolybdic acid supported on silica gel15, sulfonic acid on silica gel16, ammonium chloride17, p-dodecyl benzenesulfonic acid18, and Zn(NO3)219 among others. Previously, we have synthesized a number of heterocyclic compounds20-30.
In this study, we have used of perchlorateas a catalysts to develop a new and easy methodology for the synthesis of xanthene derivatives. The experiments were started with the study of one pot reaction, available time reaction with high yields, easy separation of product, and a 3-component method, mild and efficient method for the preparation of the xanthenes (Scheme1).
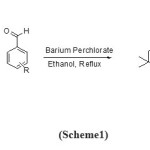 |
Scheme 1 Click here to View Scheme |
Experimental
All chemicals were obtained from Merck or Fluka without further purification. Silica gel SILG/UV 254 plates were used for TLC. IR spectra were measured on a Shimadzu IR-470 Spectrophotometer. 1H NMR spectra were determined on Bruker 400 DRX AVANCE instrument at 400 MHz, respectively.
General procedure for preparation of A1
A mixture of benzaldehyde (1 mmol), dimedone (2 mmol), barium perchlorate (15 mol %) as a catalyst with 10 ml ethanol as a solvent was refluxed at 3 hours. The progress of reaction was monitored by TLC. After finishing, recrystallized from ethanol 95% to give pure products (N1)
Spectral Data
White Crystals, Yield: (91%), m.p 200-204 oC.
FT-IR (Vmax/cm-1) (KBr disc): 3000 (CH arom. Str.); 2940 (CH aliph Str.); 1600 (C=O Str.); 1500 (C=C Str).
1H NMR (400 MHz CDCl3) δ (ppm) = 1.14 (6H, s, 2CH3); 1.28 (6H, s, 2CH3); 2.38-2.51 (8H, m, 4CH2); 5.55 (2H, s, CH), 7.18-7.35 (5H, m, CH arom).
Results and Discussion
We have been able to introduce an efficient and environmentally friendly for the synthesis of xanthene derivatives via condensation of dimedone with various aromatic aldehydes. Therefore, reported new catalyst which could provide an efficient, cheap, easy separation, high yield and simple route under solvent-free condition for the synthesis of 1,8-Dioxo-octahydroxanthenes.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Clark, J. H. Acc Chem Res., 2002, 35, 791-797.
- Salehi, P.; Zolfigol, M. A.; Shirini, F.; Baghbanzadeh, M. Curr Org Chem. 2006, 10, 2171-2189.
- Mohammadizadeh, M.R.; Hasaninejad, A.; Bahramzadeh, M.; Khanjarlou, Z. S. Synth Commun. 2009, 39, 1152-1162.
- Hasaninejad, A.; Zare, A.; Sharghi, H.; Shekouhy, M. Arkivoc, 2008, xi, 64-67.
- Hasaninejad, A.; Zare, A.; Balooty, L.; Mehregan, H. Synth Commun. 2010, 40, 3488 –3495.
- Iranpoor, N.; Firouzabadi, H.; Jamalian, A.; Kazemi, F. Tetrahedron, 2005, 61, 5699 -5704.
- Kalinski, C.; Lemoine, H.; Schmidt, J.; Burdack, C.; Kolb, J.; Umkehrer, M.; Ross, G. Synlett. 2008, 24, 4007–4011.
- Peet, N. P.; Huber, E.W.; Huffman, J. C. J. Heterocycl. Chem. 1995, 32, 33-38.
- Schumacher, K.; Ravikovitch, P.I.; Du Chesne, A.; Neimark, A.; Unger, K. K. Langmuir, 2000. 16, 4648 -4654.
- Chibale, K.; Visser, M.; Schalkwyk, D. V.; Smith, P. J.; Saravanamuthu, A.; Fairlamb, A. H. Tetrahedron, 2003, 59(13), 2289 –2296.
- Hideo, T.; Jpn. Tokkyo Koho JP 56005480., Chem. Abst., 1981, 95, 80922b.
- Hatamjafari, F.; Khojastehkouhi, H. Orient. J. Chem. 2014, 30, 329-331.
- Hatamjafari, F.; Germani Nezhad, F. Orient. J. Chem. 2014, 30, 355-357.
- Poupelin, J. P.; Saint-Rut, G.; Fussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.; Lakroix, R. Eur. J. Med. Chem. 1978, 13, 67-71.
- Kantevari, S.; Bantu, R.; Nagarapu, L. J Mol Catal A: Chem. 2007, 269, 53-57.
- Maghsoodlou, M. T.; Habibi-Khorassani, S. M.; Shahkarami, Z.; Maleki, N.; Rostamizadeh, M. Chin Chem Lett. 2010, 21, 686-689.
- Srihari, P.; Mandal, S. S.; Reddy, J. S. S.; Srinivasa Rao, R.; Yadav, J. S. Chin. Chem. Lett. 2008, 19, 771-774.
- Mahdavi, G. H.; Bigdeli, M. A.; Saeidi Hayeniaz, Y. Chin. Chem. Lett. 2009, 20, 539-541.
- Wang, X. S.; Shi, D. Q.; Li, Y. L.; Chen, H.; Wei, X. Y.; Zong, Z. M. Synth Commun. 2005, 35, 97-104.
- Jin, T. S.; Zhang, J. S.; Xiao, J. C.; Wang, A. Q.; Li, T. S. Synlett. 2004, 866-870.
- Vahabi, S. A. H.; Hatamjafari, F.; Pourshamsian, K. Orient. J.Chem. 2014, 30, 849-851.
- Azizian, J.; Hatamjafari, F.; Karimi, A. R.; Shaabanzadeh, M. Synthesis, 2006, 5, 765-767.
- Azizian, J.; Shaabanzadeh, M.; Hatamjafari, F.; Mohammadizadeh, M.R. Arkivoc, 2006, xi, 47-58.
- Hatamjafari, F. Synthetic Communications. 2006, 36, 3563–3570.
- Azizian, J.; Hatamjafari, F.; Karimi, A. R. Journal of Heterocyclic Chemistry, 2006, 43, 1349-1352.
- Hatamjafari, F.; Montazeri, N. Turkish Journal of Chemistry, 2009, 33, 797-802.
- Hatamjafari, F. Orient. J. Chem. 2012, 28, 141-143.
- Hatamjafari, F. Orient. J. Chem. 2013, 29, 93-95.
- Hatamjafari, F.; Alijanichakoli, F. Orient. J.Chem. 2013, 29, 145-147.
- Hatamjafari, F.; Hosseinian, A. Orient. J.Chem. 2013, 29, 109-111.

This work is licensed under a Creative Commons Attribution 4.0 International License.









