Factors Affecting the Physical Properties of Epoxidized Natural Rubber (ENR)/ Polyvinyl Chloride (PVC)Aerogels
Rizafizah Othaman,*, Yeon Hui Yuan, Muntaz Abu Bakar
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia corresponding author: E-mail address: rizafizah@ukm.edu.my
DOI : http://dx.doi.org/10.13005/ojc/310211
Article Received on :
Article Accepted on :
Article Published : 26 Jun 2015
Microsized aerogels were prepared from epoxidized natural rubber (ENR)/polyvinyl chloride (PVC) matrix blend using the solvent exchange method and dropping technique with sonication. Some parameters were manipulated in order to observe its effects on resultant aerogels. Parameter studied were ENR/PVC solution viscosity, ratio of ENR/PVC solution to EtOH/H2O solution and sonication time.It was found that the best parameter to produce spherical microsized ENR/PVC aerogels with high water absorption capability was by using the ENR/PVC to THF ratio of 1:20, ENR/PVC solution to EtOH/H2O solution of 1:4 and a sonication time of 3 minutes.
KEYWORDS:aerogels; sonication; voids; absorption
Download this article as:| Copy the following to cite this article: Othaman R, Yuan Y. H, Bakar M. A. Factors Affecting the Physical Properties of Epoxidized Natural Rubber (ENR)/ Polyvinyl Chloride (PVC)Aerogels. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Othaman R, Yuan Y. H, Bakar M. A. Factors Affecting the Physical Properties of Epoxidized Natural Rubber (ENR)/ Polyvinyl Chloride (PVC)Aerogels. Available from: http://www.orientjchem.org/?p=9521 |
Introduction
One of the wildly researched materials nowadays due to its interesting physical properties is aerogel. Aerogel is a unique solid material which has low density with more than 95% of its volume consisting of air. It also has large open pores and a high inner surface area. These properties result in extremely low thermal conductivity and low sound velocity apart from high optical transparency(1). Aerogels are used in a variety of applications mainly as insulators. Some of its other applications include chemical absorber, catalyst or catalyst carrier, thickening agent, and as a drug delivery system(2). Aerogels are prepared using various kinds of methods namely emulsion polymerization (3-5), emulsion with solvent evaporation (ESE) (6-8), precipitation polymerization (9), and ambient pressure drying (2).
In this study, microsizedaerogels was prepared from a thermoplastic elastomer consisting of epoxidized natural rubber (ENR) blended with polyvinyl chloride (PVC) using the solvent exchange method, dropping and sonication process.ENR is considered a good elastomer because of its enhanced properties including flexibility, hydrophilicity and high shearing. PVC, on the other hand is an outstanding material with properties such as excellent chemical and physical properties, low cost, high stiffness and robustness. ENR has a good interaction with PVC and is expected to provide elasticity and gel properties to the blend whilst PVC provides mechanical strength(10-12).Some parameters were manipulated during the preparation process in order to observe the effects on the formation of aerogels and it absorption properties.
Materials and Methods
Materials
ENR of 50% epoxidation (ENR-50) with a molecular mass of 670, 000 g/mol was supplied by the Malaysian Rubber Board. Industrial grade PVC was supplied by Sigma-Aldrich while tetrahydrofuran (THF) and ethyl alcohol (EtOH) was provided by Systerm.
Aerogel Preparation
The ENR/PVC blend was prepared by melting 24 g of ENR with 36 g of PVC inside an internal mixer (Brabender Plasticoder PL2000) to produce a 40:60 (w/w) ENR/PVC composite. The ENR/PVC was then dissolved in THF for 24 h before being stirred for another 24 h using magnetic stirrer. In this study, the ratio of ENR/PVC to THF volume used was 1:7, 1:10 and 1:20 w/v.
ENR/PVC solution was then sonicated using an ultrasonic sonicator (Ultrasons MEDI-II). EtOH/H2O mixture solution was continuously dropped into the ENR/PVC solution with 10 drops/min rate. Sonication time was varied at 1, 3, and 5 minutes. The sonication process was conducted with the aim of agitating the intermolecular interaction of the ENR/PVC matrix thus allowing better interaction of theEtOH/H2O solution with the matrix surfaces.
The volume ratio of ENR/PVC solution to EtOH/H2O solution was then variedas 1:1, 1:2, 1:4 and 2:1.After that, the solution was filtered using a Buchner funnel to collect wet gels formed. The wet gels were then dried in an oven at 80 °C for 3 hours to produce aerogels. All the parameters studied are tabulated in Table 1.
Table1: Parametersunder study in the preparation of aerogels from ENR/PVC
| Parameter |
Sample |
ENR/PVC:THF (mass:vol) |
ENR/PVC solution: EtOH/H2O solution (vol:vol) |
Sonication time (min) |
| 1) Concentration of ENR/PVC solution |
a |
1:7 |
1:4 |
3 |
|
b |
1:10 |
1:4 |
3 |
|
|
c |
1:20 |
1:4 |
3 |
|
| 2) Ratio of ENR/PVC and EtOH/H2O solution |
d |
1:20 |
1:1 |
3 |
|
e |
1:20 |
1:2 |
3 |
|
|
f |
1:20 |
1:4 |
3 |
|
|
g |
1:20 |
2:1 |
3 |
|
| 3) Sonication time |
h |
1:20 |
1:4 |
1 |
|
i |
1:20 |
1:4 |
3 |
|
|
j |
1:20 |
1:4 |
5 |
Characterization
The morphology of all aerogels was observed using scanning electron microscopy (SEM) (LEO 1450 VP). The samples were coated with aurum using sputter coater before images were taken. Aerogels were also tested for water absorption. A mass of dried aerogels was taken before they were soaked in water for 24 hours. Calculation of water absorption was performed using Eq. 1.
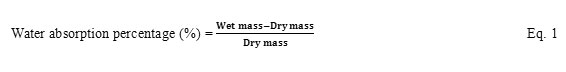
Results and Discussion
Effect of ENR/PVC Solution Concentration on Aerogel Morphology
Figure 1aillustrates the SEM micrograph of aerogels prepared with parameters as in (a) in Table 1. It showed that the size of the aerogel beads was between 2-7µm.Voidspaces was also observed between the aerogel beads. At high concentrations of ENR/PVC solution, the effect of sonication was not strong enough to perfectly break the solution into beads. As reported by Abdi et al. (13), higher energy was needed to break a solution with higher concentration into beads.
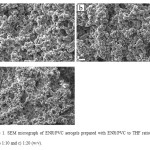 |
Figure1: SEM micrograph of ENR/PVC aerogels prepared with ENR/PVC to THF ratio of a) 1:7, b) 1:10 and c) 1:20 (w/v). |
Aerogels prepared with the ratio of ENR/PVC to THF being 1:10 w/v is shown in Figure 1b. The SEM image showed that the shape of the beads were more uniform with the size being <5 µm. More voidspaces were also observed between the aerogel beads as compared to 1a. Some aerogel beads were observed to stick between each other. This might have happened due to the uneven spread of newly formed beads with other beads during the sonication process thus resulting in agglomerated beads.
Figure 1c shows the aerogels prepared with the solvent ratio 1:20 w/v. At 2000× magnification, the aerogel beads were observed to be spherical with sizes of < 3 µm. Similar to Figure 1b, voidspaces were observed between the aerogel microbeads. Smaller bead sizes were the result of lower concentrations of the ENR/PVC solution making it easier for the sonication process to break the solution into microbeads.
Effect of the Ratio of ENR/PVC Solution to EtOH/H2O on Aerogel Morphology
Figure 2 demonstrates the SEM images of aerogels prepared by changing the ratio of ENR/PVC solution to EtOH/H2O solution. All images were 2000× magnified. Figure 2a shows the surface of aerogels prepared with details as in (d) in Table 1 where the ratio used was 1:1 v/v followed by figures 2b. 1:2, 2c.1:4 and 2d. 2:1. All the aerogels appeared to be similar with no significant differences when observed through SEM with microbead sizes being less than 3 mm. In a research conducted by Alnaief and Smirnova (3),the micro sized aerogels produced had different sizes when the ratio of the spreading phase to continuous phase was changed. By increasing the composition of the discontinuous phase, smaller bead sizes were produced. It was different for this case as the EtOH/H2O solution did not influence the size of the microbeads and only acted as a solidifying agent in forming gels.
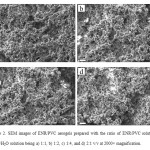 |
Figure2: SEM images of ENR/PVC aerogels prepared with the ratio of ENR/PVC solution to EtOH/H2O solution being a) 1:1, b) 1:2, c) 1:4, and d) 2:1 v/v at 2000× magnification. |
Effect of Sonication Time on Aerogel Morphology
Sonication time of ENR/PVC solution was varied to observe its effects on aerogels produced. SEM images in Figure 3 illustrate the aerogels produced with a sonication process of 1, 3, and 5 minutes. Other parameters are as tabulated in Table 1. Figure 3a shows that the size and shape of the aerogels produced cannot be determined. This is because 1 minute is not enough for the sonication process to break the ENR/PVC solution into micro-sized gels. SEM images of aerogels with 3 and 5 min sonication times were observed to be spherical in shape with almost uniform sizes of < 5 mm. However, voidspaces in aerogels with a 5 minute sonication time were less than aerogels with a 3 minute sonication time and the structure was denser. This could be the result of longer sonication time which interfered with the aerogels stacking. Thus, the optimum sonication time is 3 minutes.
 |
Figure3: SEM images of ENR/PVC aerogels prepared with sonication time of ENR/PVC solution in a) 1 minute, b) 3 minutes and c) 5 minutes at 2000× magnification. |
Water Absorption Test
The results of the water absorption test showed that the lower the ratio of ENR/PVC to THF (w/v), the higher the water absorption percentage. This is as shown in Figure 4 where with a ratio of 1:7, the aerogels only absorbed 37.7% water which was the lowest. This could be explained by the inefficiency of solvent exchange between the THF and EtOH/H2O due to the low THF volume. By referring to the earlier SEM image (Figure 1a), because of its shape which was not uniform, there was less surface area,and therefore affected the surface contact with water.
On the other hand, aerogels prepared with ENR/PVC to a solvent ratio of 1:10 could absorb water at almost its dry mass. Meanwhile, aerogel prepared with a solvent ratio of 1:20 absorbed water more than its dry mass proving that the more solvents are used, the more voidspaces will be present between resultant aerogel beads as discussed in the SEM images.
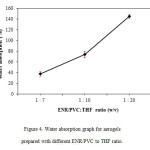 |
Figure4: Water absorption graph for aerogels prepared with different ENR/PVC to THF ratio. |
Water absorption results for aerogels with different ratios of ENR/PVC solution to EtOH/H2O solution (v/v) are shown in Figure 5. As discussed earlier, the change in this ratio did not significantly affect the shape and size of the aerogels produced. However, by performing water absorption tests, it was found that the lower the ratio of ENR/PVC solution to EtOH/H2O solution, the higher the water absorption percentage.
From Figure 5, it can be seen that aerogels prepared with 1:4 ratio of ENR to EtOH/H2O solution possessed the highest water absorption percentage,which was four times higher than the water absorption of aerogels prepared with 2:1 ratio. For aerogels prepared with 1:1 and 1:2 solution ratios, the difference between the two was not significant (about 1%) but the water absorption percentages recorded were more than one fold of their dry mass. This phenomenon could be the result of increasing void formation with increasing EtOH/H2O solution used which could not be observed on the surface of the aerogels (14).
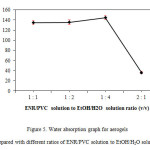 |
Figure5: Water absorption graph for aerogels prepared with different ratios of ENR/PVC solution to EtOH/H2O solution. |
Results on the water absorption test for aerogels prepared with different sonication time is represented in Figure 6. From the figure, when sonication time increases, the water absorption percentage of aerogels also increases with a slight decrease in the 5 minute sonication time. It appears that aerogels prepared with a 3 minute sonication time absorbed more water than the other two sonication times. This is consistent with morphological observation by SEM earlier where aerogels with a 3 minute sonication time appeared to have more voidspaces than others.
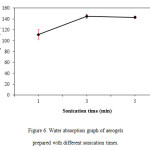 |
Figure6: Water absorption graph of aerogels prepared with different sonication times. Click here to View figure |
Conclusion
Aerogels were successfully prepared from the ENR/PVC matrix using solvent exchange and dropping with sonication techniques. The resultant aerogels were micro in scale and spherical in shape with observable voidspaces under SEM microscope. Their morphology and water absorption properties were observed to be affected by the different parameters used during the preparation process. Aerogels with the best properties were the ones prepared with the ratio of ENR/PVC to THF being 1:20 (w/v), ratio of ENR/PVC solution to EtOH/H2O solution being 1:4 (v/v) and a sonication time of 3 minutes.
References
- Hüsing, N., Schubert, U. Angew. Chem. Int. 1998,37, 22-45
- Aravind, P.R., Shajesh, P., Soraru, G.D., Warrier, K.G.K. J. Sol-Gel Sci. Technol. 2010,54, 105-117
- Alnaief, M., Smirnova, I. J. Supercrit. Fluids. 2011,55, 1118-1123
- García-González, C.A., Alnaief, M., Smirnova, I. Carbohydr Polym. 2011,86, 1425-1438
- Liu, M., Gan, L., Pang, Y., Xu, Z., Hao, Z., Chen, L. Colloids Surface A 2008,317, 490-495
- Ito, F., Fujimori, H., Makino, K. Colliods Surface B 2007,54, 173-178
- Kim, H.K., Chung, H.J., Park, T.G. J. Control. Release 2006,112, 167-174
- O’Donnell, P.B., McGinity, J.W. Adv. Drug Deliv. Rev. 1997,28, 25-42
- Karagoz, B., Bayramoglu, G., Altintas, B., Bicak, N., Yakup Arica, M. Bioresour Technol. 2011,102, 6783-6790
- Ibrahim, A., Dahlan, M. Prog. Polym. Sci. 1998,23, 665-706
- Jon, N., Samad, N.A., Abdullah, N.A., Abdullah, I., Othaman, R. J. Appl. Polym. Sci. 2013,129, 2789-2795
- Abdullah, N.A., Abdullah, I., Othaman, R. Int. J. Materials Engineering Innovation 2013,4, 269-280
- Abdi, S., Ng, S., Choi, J., Seo, J., Lim, J. Macromol. Res. 2010,18, 668-673
- Wu, G., Yu, Y., Cheng, X., Zhang, Y. Mater. Chem. Phys. 2011,129, 308-314

This work is licensed under a Creative Commons Attribution 4.0 International License.









