Design and Manufacture of Silver-Selective Electrode Based on Single-Walled Carbon Nanotubes
Khoorshid Mehdizadeh1, Masoud Giahi2*, Hossein Aghaie3, Majid Monajjemi3
1Science and Research Branch, Islamic Azad University, Tehran, Iran.
2Lahijan Branch, Islamic Azad University, Lahijan, Iran.
3Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/ojc/310212
Article Received on :
Article Accepted on :
Article Published : 01 May 2015
The present research explores the design and manufacture of coated ion-selective membranes on graphite electrodes with selectivity towards silver cations.Single- walled carbon nanotube N-6- aminohexylamide ,was used as the ionophore.The electrode was manufactured in a concentration range of 1×10-6to1×10-2 , , resulting in a Nernst response with a gradient of 59.1±0.5 mv/decade . In this work, the effects of membrane composition, pH of the solution, temperature and non-aqueous environment were investigated on the performance of the electrodes Furthermore, the response time of the electrode and the electrode response reversibility were calculated using both static and dynamic methods. This electrode can be applied in ethanol environments of up to 25% and in dioxane environments of up to 25% volume-volume without hindrance. The electrode response time was less than 18 seconds.
KEYWORDS:Carbon Nanotube; Ion-Selective; Graphite Electrode; N-6-Aminohexylamide
Download this article as:| Copy the following to cite this article: Mehdizadeh K, Giahi M, Aghaie H, Monajjemi M. Design and Manufacture of Silver-Selective Electrode Based on Single-Walled Carbon Nanotubes. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Mehdizadeh K, Giahi M, Aghaie H, Monajjemi M. Design and Manufacture of Silver-Selective Electrode Based on Single-Walled Carbon Nanotubes. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8723 |
Introduction
Silver is a metallic, silver-colored element which is quite rare and expensive. Silver salt compounds are toxic. Free silver ion with a concentration of 1-5 micrograms per liter is harmful to aquatic plant species, invertebratesandboney fish and if its concentration reaches 0.17 micrograms, it can cause difficulties in the growth of salmon. Concentrations of 0.3 – 0.6 micrograms per liter are fatal for phytoplankton species. This ion enters the industrial effluents due to the corrosion of internal surfaces of pipes and generators. Ion-selective membranes have many applications in the measurement and identification of various compounds including drugs and environmental contaminants as well as water and waste water treatment. Today, lots of these ion-selective membranes have been commercialized as electrodes which are used for measuring different combinations.
Nowadays, the applications of nanotechnology in scientific and industrial fields are commonly known.The first single-walled carbon nanotube (SWNTs) was made in 1993, by IijimaandDonaldBethune simultaneously and independently. Single-walled carbon nanotubes usually have a diameters between 1 – 1.5 nanometers and a length of about a few microns. This type of nanotubes is stronger than multi-walled carbonic nanotubes (MWNTs); because in MWNTs, the links between the internal plates are weak. A carbon nanotube (NTC), due to the curvature and arched shape of its surface, has better reactivity compared to a graphite plate. The reactivity of carbon nanotubes depends on the unbalance of their orbital which occurs due to surface curvature. Therefore, nanotubes with less curvature have more reactivity. Depending on the arrangements of the carbon atoms, nanotubes have different shapes. Armchair carbon nanotubes (n,n) are 1000 times more conductive than copper, while the zigzag type (n,0) and asymmetrical type (n,m) are semiconductors.The present research reports the design and manufacture of coated ion-selective membranes on graphite electrodes with selectivity towards the silver cation. The carbon nanotubes are presented in Fig. 1.
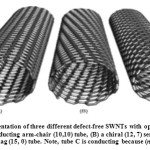 |
Figure1: An idealized representation of three different defect-free SWNTs with open ends with lattice vectors (A) an achiral metallic conducting arm-chair (10,10) tube, (B) a chiral (12, 7) semi-conducting tube and (C) an achiral conducting zig-zag (15, 0) tube. Note, tube C is conducting because (n−m)/3 is a whole number. Click here to View figure |
The first application of nanotubes in electrochemistry was introduced by Britto et al. Since this study, several papers have demonstrated the electrochemical properties of carbon nanotubes and have shown them to be equal or superior to many other electrodes.
Experimental
Ionophore-Synthesis
In order to synthesize the ionophore, 0.1 g of carboxyl-bearing single-walled carbon nanotube (SWNTS-COOH)was combined with0.1162 g of 1-6-diaminohexane and the mixture was kept at room temperature for 48 h after adding 5 ml of distilled water. Then, the existing water was evaporated using a heater and the ionophore was dried. The Fourier Transform Infrared Spectroscopy (FTIR)of the carboxyl-bearing single-walled carbon nanotubes and ionophore are shown in Fig. 2 and Fig. 3.A solution to spectroscopy was the use of KBr preparations of nanotube samples.
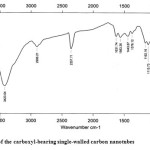 |
Figure2: FTIR spectrum of the carboxyl-bearing single-walled carbon nanotubes Click here to View figure |
The peak at 3423.04 can be assigned to the O-H stretch from the carboxyl groups (O=C−OH and C−OH), while the peak at 2357.71 can be associated with the O−H stretch from the strongly hydrogen-bonded −COOH.
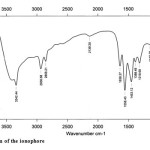 |
Figure3: FTIR spectrum of the ionophore Click here to View figure |
The peaks at 2858.21 and 2936.68 show groups and the peak at 1558.43 indicates the formation of amide, which shows N−H and N−C bands and a N−C=O group. The absorption in the 3342.44 area shows the loss of the carboxyl group and shows a group.
Results and Discussion
Optimization of the Membrane Components
The membrane is a type of paste, composed of %30.36 graphite powder and %6.07 ionophore in %63.57 paraffin oil. The hole related to Teflon mold was filled with the prepared paste; in a way that the paste could touch the graphite electrode from one side and the solution under experiment from the other side. The height of the mentioned cavity is 2 mm and its diameter is equal to the outer diameter of the graphite electrode (6 mm). After drying for 2 h, the mentioned membrane gave constant and stable responses with gradients of . Results have been presented in table 1 and Fig. 4.
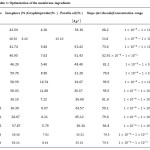 |
Table1: Optimization of the membrane ingredients
|
 |
Figure4:The Teflon mold related to the graphite electrode, in both states of empty and filled with the paste related to membrane. Click here to View figure |
Measurement of Electromotive Force (EMF)
A Saturated Calomel Electrode (SCE) was used as the external reference electrode. pH of the prepared solutions was measured using a pH meter device at 25.0 ± 0.1 . The electrode was made in a concentration range of 1.0×10-6 to 1.0×10-2 of silver ion (Ag+) and a pH between 3 to 6, which gave a Nernst response with gradient of 59.1± 0.5 . The results have been presented in table 2 and Fig. 5.
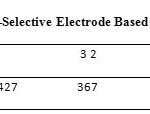 |
Table2: Potential Changes of Silver Ion-Selective Electrode Based onpM Click here to View table |
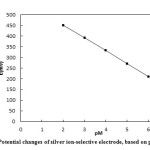 |
Figure5: Potential changes of silver ion-selective electrode, based on pM Click here to View figure |
Investigation of the Effect of pH on Electrode Response
To investigate the effect of pH, a 1.0×10-3 silver ion solution was selected and concentrated HNO3 was used to adjust the pH of the solutions between 1 and 7 (In order to produce a solution with pH = 7, distilled water was used). Then, the potential of each solution was recorded by a digital multimeter. Based on Fig. 6, the response of the electrode in pH range of 3 to 6 is independent of the change in pH. In solutions with pH less than 3, the electrode response depends on pH, due to the protonation of ionophore. Table 3.shows the potential changes of silver ion-selective electrodes based on pH.
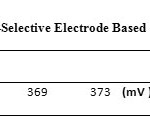 |
Table3: Potential Changes ofSilver Ion-Selective Electrode Based on pH Click here to View table |
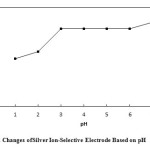 |
Figure6: Potential Changes of Silver Ion-Selective Electrode Based on pH Click here to View figure |
Investigation of the Response Time of Ion-Selective Electrode
The response time of the suggested electrode was studied by both static and dynamic methods. In the static method, 1.0×10-3 and 1.0×10-4 of silver ion solutions were used. Since the dynamic response time is an important factor for an ion-selective electrode, it was calculated as follows. In the dynamic method, a=1.0×10-5, b=5.0×10-5, c=1.0×10-4, d=5.0×10-4, e=1.0×10-3 and f=5.0×10-3 of silver ion solutions were used. The potential was recorded every 3 seconds. The results have been presented in the form of a chart showing E (mv) against t (s) (Fig. 7 and Fig. 8 ). The response time of the electrode in both methods was s≥18.
 |
Figure7: The static response time of the electrode for step changes in the concentrations of Ag+ Click here to View figure |
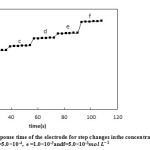 |
Figure8: The dynamic response time of the electrode for step changes inthe concentration of Ag+ : a=1.0×10-5, b= 5.0×10-5, c =1.0×10-4, d =5.0×10-4, e =1.0×10-3andf=5.0×10-3 Click here to View figure |
Reversibility Investigation of the Response of Ion-Selective Electrode
Potentials of 1.0×10-4 and 1.0×10-3 solutions of silver ion were measured 5 times in succession (low_to_high). Fig. 9 shows that the suggested electrode has a good reproducibility.
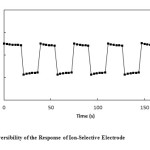 |
Figure9: Reversibility of the Response of Ion-Selective Electrode |
Study of the Effects of Organic Solvents on the Response of Silver Ion-Selective Electrode
Performance of silver ion-selective electrodes in environments containing mixtures of water-ethanol and water-dioxane was evaluated. The presence of ethanol up to %25 and dioxane up to %25 does not make a significant impact on the performance of the electrode; but since the dipole moment of dioxane (3.06), compared to ethanol (1.69) is more than water (1.82 ), in concentrations lower than dioxane, the environment around the ion changes and its effective concentration is reduced at low concentrations. The results have been presented in table 5.
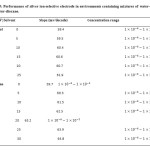 |
Table5: Performance of silver ion-selective electrode in environments containing mixtures of water-ethanol and water-dioxane. Click here to View table |
Refrences
- Aghaie, H., Giahi, M., Monajjemi, M., Arvand, M., Nafissi, G.H., and Aghaie, M., Sens Actuators B, 2005, vol. 107, p. 756.
- Aghaie, M., Giahi, M., Aghaie, H.,Arvand, M., Pournaghdy, M., and Yavari, F., Desalination, 2009, vol. 251, p. 346.
- J.J. Gooding, R. Wibowo, J.Q. Liu, W.R. Yang, D. Losic, S. Orbons, F.J. Mearns, J.G. Shapter, D.B. Hibbert, J. Am. Chem. Soc. 2003,125,9006.
- Aghaie, M., Giahi, M., Aghaie, H., and Etryan, A., Russian Journal of Electrochemistry.2009, vol. 45, p. 804.
- Arvand, M., Pourhabib, A. Shemshadi, and Giahi, M., Anal. Bioanal. Chem., 2007, vol. 387, p. 1033.
- Giahi, M., Aghaie, H., Arvand, M., and Hejri, A.M., Russian Journal of Electrochemistry, 2005, vol. 41, p. 1290.
- Giahi, M., Arvand, M., Mirzaei, M., Bagherinia, M.A., Anal. Lett.,2009, vol. 42, p. 870.
- Giahi, M., Pournaghdy, M., and Rakhshaee, R., J. Anal. Chem., 2009, vol. 64, p. 188.
- Giahi, M. Mirzaei and M. Veghar, G., J. Iranian Chem. Soc., 2010, vol. 7, p. 333.
- Giahi, M. .Marvib, O. Safaric ,F.and B. Chahkandid, B . , G., J. Iranian Chem. Soc., 2013, vol. 68, No. 102013.
- Wohlstadter, J.N.Wilbur, J.L.Sigal, G.B.Biebuyck, H.A.Billadeau, M.A.Dong, L.W.Fischer, A.B. Gudibande,S.R. Jamieson, S.H. Kenten,J.H.Leginus, J.Leland, J.K.Massey, R.J. Wohlstadter, S.J. Adv. Mater. Soc., 2003, vol. 15, No. 1184.
- Hirsch, A. Angew. Chem. Int. Edit.2002, vol. 41, p. 1853.
- Iijima, S. Nature 1991, vol. 56, p. 354.
- Guiseppi-Elie, A. Lei,C.H. Baughman, R.H. Nanotechnology. 2002, vol. 13, p. 559.
- Callegari,A. Cosnier,S. Marcaccio, M. Paolucci, D.Paolucci, F. Georgakilas, V.Tagmatarchis, N.Vazquez, E. Prato, M. Mater. J. Chem. Soc., 2004, vol. 14, p. 807.
- Liu, L.Qin, Y.Guo, Z.-X. Zhu, D. Carbon.Soc.2003, vol. 41, No. 102013.
- McCreeryR.L.Electroanalytical Chemistry, Dekker,New York, 1991.vol. 17.

This work is licensed under a Creative Commons Attribution 4.0 International License.









