Correlation between inhibition efficiency and chemical structure of new indolo imidazoline on the corrosion of mild steel in molar HCl with DFT evidences
Sowmya Ramkumar and D. Nalini*
Department of Chemistry, PSGR Krishnammal College for Women, Coimbatore – 641004, Tamil Nadu, India.
DOI : http://dx.doi.org/10.13005/ojc/310255
Article Received on :
Article Accepted on :
Article Published : 12 Jun 2015
The present work aims at the synthesis, characterization and study on the inhibitive effect of indoloimidazoline derivative (DI) on mild steel in 1M HCl. Weight loss measurement and electrochemical AC and DC corrosion monitoring techniques are performed at 308 K using mild steel specimens immersed in 1M HCl in the presence and absence of DI. Polarisation curves indicated that DI acted as a mixed type inhibitor. The indoloimidazoline derivative having nitrogen atoms in its molecular structure are adsorbed on the metal surface through these active centres and that the extent of inhibition is directly related to the formation of the adsorption layer according to Langmuir isotherm model on the mild steel surface which is sensitive function of the molecular structure. Quantum chemical calculations performed using DFT B3LYP 6-31G (d, p) basis set within the program Gaussian 09 showed the adsorption sites for DI on mild steel. Experimental and theoretical findings agreed well with each other.
KEYWORDS:Mild steel; EIS; Acid corrosion; Indoloimidazoline; DFT calculation
Download this article as:| Copy the following to cite this article: Ramkumar S, Nalini D. Correlation between inhibition efficiency and chemical structure of new indolo imidazoline on the corrosion of mild steel in molar HCl with DFT evidences. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ramkumar S, Nalini D. Correlation between inhibition efficiency and chemical structure of new indolo imidazoline on the corrosion of mild steel in molar HCl with DFT evidences. Available from: http://www.orientjchem.org/?p=9248 |
Introduction
Corrosion is a serious environmental problem in the oil, fertilizer, metallurgical and other industries. Metals are prone to corrode when they are exposed to aggressive media such as acids, bases and salts. Hydrochloric acid is a strong inorganic acid that is used in many industrial processes like acid pickling, acid descaling and oil well acidizing. During these processes, metals are subjected to serious acid corrosion and hence, there is always a need to protect the metal against corrosion (1). The use of inhibitors has been found to be one of the best options available for the protection of metals against corrosion in acid pickling industries. The presence of hetero atoms and suitable functional groups can facilitate the adsorption of the inhibitor on the metal surface (2). Nitrogen containing compounds are more effective for HCl, whereas sulphur containing compounds are preferred for sulphuric acid. These compounds can adsorb on metal surface which lead to decrease in the corrosion rate. Literature review shows that many N-heterocyclic compounds, such as imidazoline, triazole, pyrimidine, pyrrole, pyridine derivatives, have been used for the corrosion inhibition of iron or steel in acidic media. Even though imidazoline are easily accessible and known for many decades, the heterocyclic compounds containing N moiety embedded in a five-membered ring were introduced to the corrosion literature only recently (3,4). The present work is aimed to study the effect of new synthesized indoloimidazoline derivative for inhibition of the corrosion of mild steel in 1M HCl by using technique such as weight loss, potentiodynamic polarization and electrochemical impedance (EIS). Density functional theory was used to calculate some quantum chemical parameters for the corrosion system which allow determining the possible anchoring site suitable for the inhibitor to bond with steel surface.
Experimental Work
Synthesis and characterization of the inhibitor
3,4-dihydro-2-(4-dimthylaminostyryl)imidazo[4,5-b]indole
(DI) was synthesized as per the previously reported procedure (5) under microwave irradiation and its molecular structure is shown in Figure 1. The synthesized compound was characterized using FT-IR spectra.
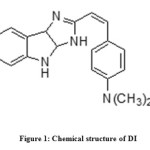 |
Figure1: Chemical structure of DI |
The band at 1360.84 cm-1 corresponds to the C-N stretching whereas the band at 1598.41 cm-1 is due to the C = N stretching. 1697.28 cm-1 band is due to the >C = C< stretching, the band at 3287.61 cm-1owe to the N-H stretching band for the secondary amine and the band at 2810.41 cm-1 corresponds to the C-H stretching.
Materials
Mild steel coupons (composition: 0.09% P; 0.38% Si; 0.01% Al; 0.05% Mn; 0.21% C; 0.05% S and the rest iron) of size 5cm x 1.5 cm x 0.5 cm were used for gravimetric measurements and for electrochemical measurements mild steel disc of geometric area 1 cm2 embedded in a Teflon coating was used The electrodes were prepared by following ASTM standard procedure (6). The aggressive solution (1M HCl) was prepared by dilution of analytical grade HCl solution with double distilled water.
Methods
Gravimetric Measurement
Gravimetric measurements were carried out in a solution volume of 100ml. The immersion period was varied from ½ h to 24 h at 303K and for 1h at higher temperatures. The concentration range of DI prepared and used was 2 – 20 mgL-1. After the corrosion test, the mild steel coupons were carefully washed in double distilled water, dried and then weighed. Weight loss allowed us to calculate the corrosion rate in g m-2 h-1.
Electrochemical Measurements
Electrochemical measurements were carried out using IVIUM Model 2723 in a three electrode electrolysis glass cell. The working electrode is a disc cut from the mild steel of 1 cm2 area embedded in a Teflon coating. A saturated calomel electrode and a platinum electrode were used as a reference and a counter electrode respectively. The potentiodynamic tafel measurements were carried out from the cathodic to anodic direction with a scan rate of 1.66 mV/ sec. EIS measurements were carried out using AC signal of amplitude 25 mV peak to peak in the frequency range of 10 kHz to 0.01 mHz at open circuit potential (OCP).
Computational details
All geometry optimizations and quantum chemical calculations were performed using density functional theory (DFT) utilizing the 6-31G (d, p) basis sets within the programme package Gaussian 09 (7). The Becke’s Three Parameter Hybrid Functional using the Lee-Yang-Parr correlation functional theory (B3LYP) was selected for the calculations. DFT/B3LYP is recommended for the study of chemical reactivity and selectivity in terms of the frontier molecular orbitals and other molecular properties discussed in terms of the Koopman’s theorem (8).
Result and Discussion
Gravimetric Tests
Weight loss measurement for mild steel in acidic medium at various immersion periods in the absence and presence of various concentrations of DI is summarized in Table 1. It is clear that the inhibition efficiency (%) increases with increasing inhibitor concentration while corrosion rate decreases with increasing inhibitor concentration (9). From the weight loss data in Table 1, it is evident that maximum % was observed at 20 mg L-1 of DI in 1M HCl (95%) and further increase in inhibitor concentration did not cause any increase. There is an increase in the surface area covered by the adsorbed molecules on the metal surface with the increase in the inhibitor concentration. The effectiveness of DI is due to the presence of N donors as well as the presence of π-electrons in benzene ring (10).
Table1: The effect of DI concentration on the corrosion rate for mild steel corrosion in 1M HCl for various immersion period at 303 K.
|
Conc mg/L |
½ h |
1 h |
3 h |
6 h |
12 h |
24 h |
||||||
|
CR gm-2 h-1 |
IE % |
CR gm-2h-1 |
IE % |
CR gm-2h-1 |
IE % |
CR gm-2h-1 |
IE % |
CR gm-2h-1 |
IE % |
CR gm-2h-1 |
IE % |
|
|
Blank |
3.0 |
– |
5.0 |
– |
41 |
– |
45 |
– |
71 |
– |
99 |
– |
|
2 |
1.0 |
67.45 |
1.5 |
69.84 |
11 |
73.64 |
8 |
82.72 |
27 |
62.70 |
45 |
54.20 |
|
6 |
0.7 |
75.36 |
1.1 |
78.66 |
9 |
79.21 |
6 |
86.11 |
24 |
66.20 |
40 |
59.62 |
|
10 |
0.6 |
79.09 |
1.0 |
80.32 |
8 |
81.59 |
5 |
88.08 |
23 |
67.33 |
37 |
62.79 |
|
14 |
0.6 |
81.00 |
0.9 |
81.99 |
6 |
85.13 |
5 |
89.57 |
22 |
68.61 |
34 |
65.26 |
|
18 |
0.5 |
83.27 |
0.8 |
83.01 |
4 |
89.73 |
3 |
93.68 |
20 |
72.34 |
30 |
69.84 |
|
20 |
0.5 |
83.27 |
0.7 |
85.32 |
3 |
91.55 |
2 |
94.99 |
18 |
74.33 |
29 |
70.65 |
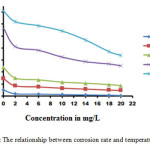 |
Figure2: The relationship between corrosion rate and temperature of DI |
From Table 1, it can be seen that the inhibition efficiency increases from 83.27 % for ½ h immersion to about 94.99 % at 6 h immersion after which it decreases to 70.65% for 24 h. This behaviour can be explained on the basis that prolonged immersion of steel in acidic solution, (i) allows the cathodic hydrogen evolution kinetics increases presumably as more cathodic sites are exposed by the corrosion process (11) and (ii) increase in the concentration of ferrous ions which is known for its stimulation of corrosion attack of the acid on the base metal. Thus DI is found to be a better inhibitor for mild steel corrosion in 1M HCl from immersion studies result (12).
Figure 2 shows the variation of corrosion rate with the inhibitor concentration at elevated temperatures. Figure 2 shows that the inhibition efficiency of DI is inversely proportional to temperature due to the increased rate of dissolution of mild steel and partial desorption of the inhibitor molecule from the metal surface with temperature (13). The highest efficiency of 85% is obtained at 303 K for 20 mgL-1 in 1M HCl which decreases to about 52% at 343 K for the same concentration. The decrease in efficiency is attributed to the increase in the solubility of the protective film and any reaction products precipitated on the surface of the metal that may inhibit the corrosion process (14).
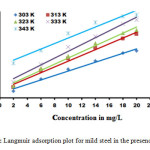 |
Figure3: Langmuir adsorption plot for mild steel in the presence of DI |
Adsorption Isotherms
In order to get a better understanding of the adsorption process on the metal surface, adsorption characteristics are studied which depends on its chemical structure (15). Adsorption isotherms are very important in determining the mechanism of organic electrochemical reactions. The most frequently used isotherms are Langmuir, Tempkin, Frumkin, Flory – Huggins, El – Awady, Freundlich and Bockris – Swinkels. In HCl, the organic compound follows the Langmuir adsorption isotherm which is described by the equation:

Rearranging the equation gives
![]()
The plot of C/θ vs C gave straight lines (Figure 3) and the values of the regressive coefficient is almost close to unity which confirms that the adsorption of the inhibitor molecule on the mild steel surface follows Langmuir adsorption isotherm (16).
mild steel in 1M HCl solution containing DI
Electrochemical Studies
Electrochemical impedance studies
Figure 4.b shows a typical set of Nyquist plot in terms of the equivalent circuit (Figure 4.a) for mild steel in 1M HCl in the absence and presence of DI and the impedance parameters are given in Table 2. It is clear from these plots that the impedance response of mild steel has significantly changed after the addition of DI in 1M HCl. Charge transfer resistance Rct increases from 14 to 140 Ωcm2 and double layer capacitance Cdl decreases from 54 to 40μF/cm2 with the increase of DI concentration. Cdl decreases from the decrease in the local dielectric constant with increases in the thickness of the electrical double layer, signifying that DI molecules act by adsorption at the metal/solution interface (17).
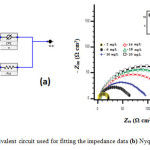 |
Figure4: (a) Equivalent circuit used for fitting the impedance data (b) Nyquist diagram |
Table2: Electrochemical parameters for the corrosion of mild steel in 1M HCL in the presence of different concentration of DI
| Electrochemical parameters | Concentration (mg/L) | |||||||
| Blank | 2 | 6 | 10 | 14 | 18 | 20 | ||
| Electrochemical impedance | Rct Ω cm2 | 14 | 45 | 66 | 81 | 104 | 127 | 140 |
| Cdl μF cm-2 | 54 | 53 | 51 | 50 | 49 | 43 | 40 | |
| IE % | – | 69.34 | 79.17 | 83.06 | 86.77 | 89.11 | 90.19 | |
| Potentiodynamicpolarization | Ecorr mV | -474 | -497 | -502 | -497 | -500 | -509 | -518 |
| Icorr μA /cm-2 | 411 | 191 | 147 | 111 | 86 | 63 | 51 | |
| ba mV | 55 | 67 | 69 | 68 | 73 | 85 | 93 | |
| bc mV | 121 | 125 | 126 | 118 | 116 | 110 | 107 | |
| IE % | – | 53.53 | 64.23 | 72.99 | 79.08 | 84.70 | 87.62 | |
Analysing the shape of the Nyquist plots, shows that the corrosion process was mainly charge transfer controlled. The general shape of the curves is very similar throughout the whole concentrations, indicating that there is no change in the corrosion mechanism occurred due to the inhibitor addition (18). The diameter of Nyquist plots increases on increasing the inhibitor concentration. These results suggest the inhibition behaviour of DI on corrosion of mild steel in 1M HCl solution.
Potentiodynamic polarization
The potentiodynamic polarization curves for mild steel in 1M HCl containing DI are shown in Figure 5 and the electrochemical parameters are given in Table 2. Inspection of this figure shows that the addition of DI has a pronounced effect on both the anodic and cathodic part of the polarization curves. This indicates that the DI can be classified as mixed type inhibitor.
 |
Figure5: Polarization curves for mild steel in 1 M HCl solution containing DI |
The slope of the Tafel lines (ba and bc) were appreciable shifted in presence of DI. The data revealed that the corrosion current density (icorr), obtained by extrapolation of the anodic and cathodic Tafel lines to the corresponding corrosion potential, decreases with increasing inhibitor concentration. the Icorr values were sharply decreased in the presence of DI. The values were 411 and 51 μA cm-2 for blank and 20 mg/L DI containing media, respectively yielding a maximum efficiency of 87.62 % at 20 mg/L. No definite trend was observed in the shift of Ecorr values, in the presence of DI, indicating that the inhibitor is first adsorbed onto steel surface and therefore impedes by merely blocking the reaction sites of iron surface without affecting the anodic and cathodic reaction mechanism (19). The corrosion potentials (Ecorr) are shifted to more negative values in the presence of DI, indicating that the inhibitor had a stronger influence on the cathodic reduction reaction. The cathodic shifts of (Ecorr) and the decreases of the corresponding current densities (Icorr) with increasing inhibitor concentration can be attributed to the higher number of adsorbed inhibitor molecules on the steel surface, which entirely cover the steel surface and decrease the corrosion rate (20).
Quantum chemical studies
Equilibrium geometry structure
The quantum chemical method was used to study the relationship between molecular structure and inhibitive effect of the inhibitor. The values of bond length and bond angle in the optimized structure are presented in Table 3. It can be seen from Table 3 that the bond lengths of C-N in the shorter than the general C-N single bond (1.470 Å). The tendency of average in the bond length is indicative of a conjugation effect in the imidazole rings. The conjugation effect restricts the free rotation of C-N bonds in the imidazole rings, resulting in the rigid planar structure of the indoloimidazole segments. Naturally, DI shows a planar geometry after DFT optimization (Figure 6). It is expected from this result that DI may adsorb on the metal surface in a horizontal state by conjugation systems with π electrons as well as N atoms with lone pair electrons supporting electron donation to the vacant d-orbital of Fe atoms (21).
Table3: Bond length (Å) and bond angle (°) for the optimized neutral molecules of DI calculated at the B3LYP/6-31 G (d, p) level of theory
| Geometry parameters | Bond length (Å) | Geometry parameters | Bond Angle (°) |
| C1 = N4 | 1.343 | C20 – N4 – C1 | 105° |
| C1 – N5 | 1.424 | N4 – C1 – N5 | 111° |
| C2 – N5 | 1.371 | C1 – N5 – C2 | 105° |
| C20 – N4 | 1.386 | C2 – N32 – C22 | 105° |
| C2 – N32 | 1.370 | ||
| C22 – N32 | 1.417 |
Table4: Calculated quantum chemical indices of DI
| Compound | EHOMO (eV) | ELUMO (eV) | ∆E (eV) | μ (Debye) | χ | η | s | ω | ∆N |
| DI | -4.43 | -0.96 | 3.47 | 6.29 | 2.7 | 1.73 | 0.58 | 2.1 | 1.24 |
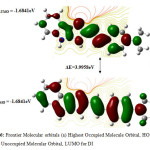 |
Figure6: Frontier Molecular orbitals (a) Highest Occupied Molecule Orbital, HOMO (b) Lowest Unoccupied Molecular Orbital, LUMO for DI |
The quantum chemical parameters of the inhibitor molecule are listed in Table 4. Higher values of EHOMO are, stronger is the electron donating ability of the molecule and lower the values of ELUMO, the more probable it is that the molecule would accept electrons. And the molecules with lower absolute values of the energy band gap (∆E = ELUMO – EHOMO) exhibit higher inhibition efficiency because the energy to remove an electron from the last x occupied orbital will be lower. The data from Table 4, high EHOMO, low value of ELUMO, energy gap, accounts for the better inhibitive property of DI (22). These are accordant with the result from experiments. With the purpose of having a wider knowledge about the local reactivity of the imidazoline derivatives, the Mulliken charge indices (Table 5), for each atom in the molecule have been calculated. An analysis of the distribution of charges and the global hardness provides a more complete scheme of the reactivity of the studied molecule. A Mulliken population analysis performed for indoloimidazoline derivatives showed that N4, N5, N32, N41 have a larger electronic density which could make them nucleophilic centres when interacting with mild steel surfaces. In Figure 6, the HOMO is localized in the imidazoline ring as well as the functional groups attached to this ring. This would indicate that the preferred actives sites for an electrophilic attack are located within the region around the nitrogen belonging to the indoloimidazoline ring. Conversely, some carbon atoms carry positive charges, which are often sites where nucleophiles can attach. Therefore, DI can also accept electrons from Fe through these atoms. It has been reported that excellent corrosion inhibitors can not only offer electrons to unoccupied orbitals of the metal but also accept free electrons from the metal (23).
Active sites for adsorption
Since the studied molecules can donate electrons to metal surface as well as accept electrons from metal surface, it is essential to examine the active sites responsible for donating or accepting electrons in the molecules. This can be achieved by an evaluation of Mulliken charge. Mulliken charge is a measurement of local reactivity as well as indicative of local nucleophilic or electrophilic feature in the molecules (24). It can be seen that for DI the N4, N5 and N32 in the indoloimidazole ring are the most susceptible sites for the electrophilic attacks as they present the highest values of negative charge. On the other hand, C1, C2, and C20 in DI are the most susceptible sites for the nucleophilic attacks as they present the highest values of positive charge. Additionally, the presence of the dimethyl amino group helps in the strong adsorption of the DI molecule on the metal surface. It can be concluded from these results that the indoloimidazole ring is the most active reaction region responsible for both donating and accepting electrons to form coordinate bonds for the adsorption of DI on iron surface. Hence DI with a planar geometry and having more adsorption sites can adsorb on the metal surface in a linear fashion (Figure 7) covering a large area. This accounts for its better inhibition efficiency for mild steel in 1M HCl.
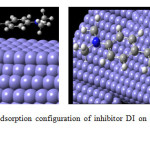 |
Figure7: Equilibrium adsorption configuration of inhibitor DI on Fe surface (a) side view (b) top view Click here to View figure |
Conclusion
- The selected indoloimidazoline derivative was found to be an effective inhibitor for mild steel corrosion in 1M HCl solutions. The inhibition efficiency of the studied inhibitors increases with decreasing in temperature and increasing of inhibitor concentration following Langmuir adsorption isotherm.
- Tafel polarization studies have shown that the selected compound suppresses both anodic and cathodic process and thus acts as mixed-type inhibitor. The results of impedance indicate that the increase in charge transfer resistance and inhibition efficiency tend with increasing the inhibitor concentration is attributed to increase of the thickness of the double layer. Thus DI inhibit corrosion by blocking the active sites.
- Theoretical calculations show that the effect of the indolo imidazoline moiety of the inhibitor is responsible for the inhibition of mild steel in an acidic chloride solution which is facilitated by the presence of phenyl rings and dimethyl amino group.
Acknowledgement
The authors acknowledge UGC, New Delhi for the financial support.
References
- Ebenso. E.E.; Obot. I.B.; Murulana. L.C.I. J. Electrochem. Sci.2010, 5 (11), 1574 – 1586
- Singh. A.K.; Quraishi. M.A. I. J. Electrchem. Sci.2012,7 (4),3222- 3241
- Dasami. P. M.; Parameswari. K.; Chitra. S. Orient. J. Chem. 2015, 31(1), 185-191
- Parameswari. K.;Rekha. S.; Chitra,; E. Kayalvizhy. Port Electrochim. Acta, 2010,28(3), 189.
- Kumar. N.; Sharma. P. K.; Garg. V. K.; Singh.P. Current Research in Chemistry,20113(2), 114 – 120
- ASTM G1-03, “Wear and Erosion: Metal corrosion”, Annual Book of ASTM Standard, ASTM, West Conshohocken, PA 30-37, (1996)
- Gaussian -Frisch MJ, Trucks GW, Schlegal HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millan JM. Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci M, Poeli C, Adamo C, Clifford S, Ochterski J, Peterson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov B, Liu G, Liashenko A, Piskorz p, Komaromi I, Gomperts R, Martin RL, Fox AJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gaussian 98.Revision A. Gaussian Inc.,Pittsburgh, PA
- Kabanda. M.;Mwadham. Sudhish K. Shukla,; Ashish K. Singh,; Lutendo C. Murulana,; Eno E. Ebenso.I. J. Electrochem. Sci.2012, 7 (9), 8813 – 8831
- Noor. E. A.; Al-Moubaraki. A.H.Mat. Chem.Phys,2008,110(1), 145 – 154
- Zarrok. H.; Zarrouk. A.; Salghi.R.; Ramli. Y.;Hammouti. B.; Assouag. M.; Essassi. E. M.; Oudda. H.; Taleb. M.J. Chem. pharma. Res,2012,4(12), 5048 – 5055
- Singh. A.K.; Quraishi. M. A.Corros. Sci,2009,51(11), 2752 – 2760
- Mayanglambam. R.S.; Sharma. V.; Singh. G.Port Electrochim. Acta,2011, 29(6), 405 – 417
- Ostovari. S.; Hoseinieh. M.; Peikari.M.; Shadizadeh. S. R.; Hashemi. S. J. Corros. Sci,2009,51(9), 1935 – 1949
- Abdel-Gaber. M.; Masoud. M. S.; Khalil. E.A.; Shehata. E. E.Corros.Sci,2009,51(12), 3021 – 3024
- Leelavathi. S.; Rajalakshmi. R.Adv. Mat. Corros,2012,1, 47 – 56
- Migahed. M. A.; Abdul-Raheim. A.M.; Atta. A. M.; Brostow. W.Mat. Chem.Phys,2010,121(1-2), 208 – 214
- Saranya. J.; Sounthari. P.; Kiruthika. K.; Saranya .G.; Yuvarani .S.; Parameswari. K.; Chitra. S. Orient. J. Chem,2014, 30(4), 1719-1736
- Khaled, K.F. Electrochim. Acta,2008, 53(9), 3484 – 3492
- Ziyi Cao.; Yongming Tang.; Hui Cang.; Jinqiu Xu.; Gang Lu.; Wenheng Jing.Corros. Sci,2014, 83, 292 – 298
- Gece. G.; Bilgiç. S.Corros. Sci,2009,51(8), 1876 – 1878
- Liu. P.Mat. Sci. Appl,2011,2(09), 1268 – 1278
- Sutiana Junaedi.; Ahmed A. Al-Amiery.; Abdulhadi Kadihum.; Abdul Amir H. Kadhum.; Abu Bakar Mohamad. I.J. Mol. Sci,2013, 14(6), 11915 – 11928
- Majid Khodaei-Tehrani.;Ali Niazi. Orient. J. Chem., 2015, 31(1), 423-429
- Larabi. L.; Benali. O.; Mekelleche. S. M.; Harek. Y.Appl. Surf. Sci,2006, 253(3), 1371 – 1378

This work is licensed under a Creative Commons Attribution 4.0 International License.









