Triterpenoid from the extract of Mangifera indica L. roots having Antispermogenic activity
Kaushik Chakraborty1, Ajay Kumar Das2, Subhash C Mandal3, Biplab De4 and Ratna Choudhury5*
1Deptt. of Pharmacology, Agartala Govt. Medical College, Agartala, Tripura, India 2Deptt. of Biochem., ICARE Institute of Medical Scs. and Research, Haldia, West Bengal, India 3Deptt. of Pharmaceutical Technology, Jadavpur University, Kolkata, West Bengal, India 4Regional Institute of Pharmaceutical Science And Technology, Agartala, Tripura, India 5Henry Derozio Academy, Kunjaban, Agartala, Tripura – 799006, India
DOI : http://dx.doi.org/10.13005/ojc/310157
Article Received on :
Article Accepted on :
Article Published : 14 Feb 2015
In vitro antispermogenic activity of an isolated triterpenoid from the extract of the root of the plant Mangifera indica L. was evaluated. The structure (3-O-acetyl-11,19-dinor-12,20-dimethyl-ursane) was confirmed by the help of different spectral analysis.
KEYWORDS:Antispermogenic; Triterpenoid; Mangifera indica L
Download this article as:| Copy the following to cite this article: Chakraborty K, Das A. K, Mandal S. C, De B, Choudhury R. Triterpenoid from the extract of Mangifera indica L. roots having Antispermogenic activity. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Chakraborty K, Das A. K, Mandal S. C, De B, Choudhury R. Triterpenoid from the extract of Mangifera indica L. roots having Antispermogenic activity. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7245 |
Introduction
In continuation of our search on antispermogenic activity1,2 and interest on Mangifera indica L. roots1, we are reporting a new pentacyclic triterpenoid having antispermigenic activity.
Different parts of the plant Mangifera indica L. are commonly used as folk medicine for a wide variety of remedies like treatment of bleeding hemorrhoids, jaundice, cough, asthma, bronchitis, fever, piles, toothache, anemia, skin disease, leprosy, anthelmintic, wounds, diabetes, urinary tract infection, rheumatism, gastric disorder, syphilis and as carminative.3-5 Many steroidal compounds and triterpenoids from Mangifera indica L. were also reported.6
Material and Methods
Roots of M. indica were collected from Agartala, Tripura in Dec, 2012 and dried under shed, powdered (100 gm obtained) and then extracted in Soxhlet apparatus (10 gm in 100 ml solvent system [Ether : Ethylacetate = 1:1]) with the aim of obtaining triterpenoid compounds. The total content of extracts was chromatographed in a glass made column (50 cm in length and 2 cm in diameter) using silica gel (60-120 mesh) as stationary phase and chloroform : methanol = 60:40 solvent system was used as mobile phase.
The structure of 3rd fraction was elucidated by the help of IR, NMR & MS. The purity of the isolated compound was confirmed by LC-chromatogram in LCMS-Shimadzu 2010A spectrophotometer as compound showed single peak at 0.573 mAbs at 254 nm.
IR spectra (KBr) was recorded on a Jasco FTIR-5300 spectrophotometer. H1-NMR and C13-NMR recorded in Bruker AC-F 300 FTNMR and Mass in Shimadzu – 2010 A. The isolated antispermogenic compound with molecular weight 470 deduced from its LC-MS APCI positive mode mass spectrum by exhibiting a molecular ionic peak at m/z 471 for [M+H]+ ion.
Compound (3rd fraction) dissolved in DMSO (1 mg/ml) was mixed to sperm volume (1 ml) in the ratio of 1:10 and sperm count motility was assessed microscopically by following standard procedure.1,2
Results and Discussion
Total five fractions were collected separately after column chromatography, concentrated, dried under reduced pressure. The collected weight of fractions from first to last were 15, 13, 22, 10 and 12 mg respectively. Among those, the 3rd fraction (22mg) showed promising antispermogenic activity, the 2nd one (13 mg) showed negligible percentage decrease in sperm count motility (13% after 30 min) and other fractions did not show any antispermogenic activity.
IR (υmax) KBr 2926 (C-H str of CH3-CO-), 2854 (C-H str), 1705 (-C=O str) cm-1. In its H1-NMR spectrum (in DMSO D6 + CDCl3 Mix) it exhibited peaks for eight tertiary methyl groups at δ 0.57, 0.69, 0.71, 0.75, 0.80 and at 0.89 ppm. Further it exhibited peak at δ 1.15 ppm for the H present in 6 membered alicyclic ring. A strong singlet at δ 2.06 might be due to a methyl group attached to a carbonyl group. Peaks at δ 2.47, 2.48, 2.49 ppm recorded due to the –CH2 and -CH of alicyclic ring adjacent to –OCO-R. A probable structure of the compound was developed as fig. 1 (3-O-acetyl-11,19-dinor-12,20-dimethyl-ursane).
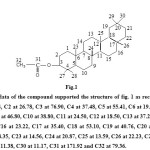 |
Figure1: The C13-NMR data of the compound supported the structure of fig. 1 as recorded in δ ppm that C1 at 38.44, C2 at 26.78, C3 at 76.90, C4 at 37.48, C5 at 55.41, C6 at 19.20, C7 at 33.62, C8 at 37.10, C9 at 46.80, C10 at 38.80, C11 at 24.50, C12 at 18.50, C13 at 37.22, C14 at 42.20, C15 at 28.86, C16 at 23.22, C17 at 35.40, C18 at 53.10, C19 at 40.76, C20 at 40.34, C21 at 28.29, C22 at 28.35, C23 at 14.56, C24 at 20.87, C25 at 13.59, C26 at 22.23, C27 at 29.86, C28 at 29.29, C29 at 11.38, C30 at 11.17, C31 at 171.92 and C32 at 79.36. Click here to View figure |
Further, mass spectra fragments confirmed the structure as in fig.1.
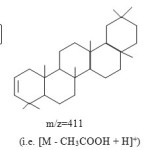 |
Figure 2 Click here to View figure |
In vitro antispermogenic activity of isolated triterpenoid compound was carried out. Percentage decrease in sperm count motility is the indicator of spermicidal activity. The isolated triterpenoid compound showed percentage decrease in motility 14%, 22% and 31% in average respectively after 10,20, 30 min.
References
- De, B.; Momin, Z. Ch.; Sen, S.; Chakraborty, R.; Goswami, B. B.; Oriental J. Chem. 2010, 26, 1067 -1069.
- Debnath, M.; De, B.; Bhattacharjee, P. R.; Choudhury, R.; Choudhury, P. S.; Advances in Pharmacol. And Toxicol. 2006, 7, 23-25.
- Anonymous, The wealth of India: A Dictionary of Indian Raw Materials and Industrial Products, Publication & Information Directorate CSIR. New-Delhi, 1st supplement series (Raw materials), Vol. 2, (2007).
- Wauthoz, N.; Balde, A.; Balde, E. S.; Damme, M. V.; Duez, P.; Int. J. Biomed. Pharm. Sci. 2007, 1, 112-115.
- Parekh, J.; Chanda, S.; Afr. J. Biotechnol. 2008, 7, 4349-4353.
- Anjaneyulu, V.; Radhika, P.; Indian J. of Chem. 2000, 39B, 883-885.

This work is licensed under a Creative Commons Attribution 4.0 International License.









