Solid Phase extraction for trace amount Cu (II) by C18 modified carbon Nano tube and cupferron ligand in waste water Sample and determination it by flame atomic adsorption.
Ali Moghimi1*, Seyed Ali Hosseini 2and Ali Mazlomi Far2
1Department of chemistry,College of Science, Varamin (Pishva) Branch, Islamic Azad University, Varamin, Iran. 2Department of chemistry,College of science, yadegar-e-Imam khomeini(RAH) shahr-e-rey Branch,Islamic Azad University, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/ojc/310129
Article Received on :
Article Accepted on :
Article Published : 11 Mar 2015
A novel, simple method has been developed for the preconcentration of Cu(II) based on the adsorption of its Schiff’s cupferron complex on a multi walled carbon nanotubes (MWCNTs) onto C18 cartridge .A new technique using a solid phase extraction (SPE) cartridge with modified Schiff’s Cupferron complex on a multi walled carbon nanotubes (MWCNTs) onto C18 cartridge as sorbent was developed for the preconcentration of trace amounts of Cu and was determined by flame atomic absorption spectrometry (FAAS). Cupferron play a key role as chelating reagent on ultrahigh specific surface of multi walled carbon nanotubes (MWCNTs). Some of the important parameters on the preconcentration and complex formation were selected and optimized. Under the optimized conditions the limit of detection (LOD) and limit of quantification (LOQ)were 0.167,0.562 and the proposed method has a good reproducibility 0.81% (RSD %).The enrichment factor was 200 and the percentage of recovery was in the range of 95-100% .The method was successfully applied to the recovery of Cu2+in different type of water samples.
KEYWORDS:Multi walled carbon nanotubes (MWCNTs); Cupferron; SPE; Preconcentration; FAAS; copper
Download this article as:| Copy the following to cite this article: Moghimi A, Hosseini S. A, Far A. M. Solid Phase extraction for trace amount Cu (II) by C18 modified carbon Nano tube and cupferron ligand in waste water Sample and determination it by flame atomic adsorption. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Moghimi A, Hosseini S. A, Far A. M. Solid Phase extraction for trace amount Cu (II) by C18 modified carbon Nano tube and cupferron ligand in waste water Sample and determination it by flame atomic adsorption. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7603 |
Introduction
The Direct determination of trace metals especially toxic metal ions such as copper, tin, copper and metalloids arsenic, antimony and selenium from various samples requires mostly an initial and efficient pre-concentration step (Leyden et al., 1976). This pre-concentration is required to meet the detection limits as well as to determine the lower concentration levels of the analyte of interest (Jones et al., 1983). This can be performed simply in many ways including liquid and solid phase extraction techniques (Nambiar et al., 1998; Caroli et al., 1991). The application of solid phase extraction technique for pre- concentration of trace metals from different samples results in several advantages such as the minimal waste generation, reduction of sample matrix effects as well as adsorption of the target species on the solid surface in a more stable chemical form(Alexandrova et al., 1993) .
The normal and selective solid phase extractors are those derived from the immobilization of the organic compounds on the surface of solid supports which are mainly nano polyurethane forms, filter paper (Leyden et al., 1975) , cellulose ( Gennaro et al., 1983) and ion exchange resins (Shamsipur et al., 2005). Silica gel, alumina, magnesia and zirconia are the major inorganic solid matrices used to immobilize the target organic modifiers on their surfaces (Unger et al., 1979) of which silica gel is the most widely used solid support due to the well documented thermal, chemical and mechanical stability properties compared to other organic and inorganic solid supports
(Boudreau et al., 1989). The surface of silica gel is characterized by the presence of silanol groups, which are known as weak ion exchangers, causing low interaction, binding and extraction of the target analytes ( Kvitek et al., 1982). For this reason, modification of the silica gel surface with certain functional groups has successfully been employed to produce the solid phase with certain selectivity characters (Bruening et al., 1991). Two approaches are known for loading the surface of solid phases with certain organic compounds and these are defined as the chemical immobilization which is based on chemical bond formation between the silica gel surface groups and those of the organic modifier, and the other approach is known as the physical adsorption in which direct adsorption of the organic modifier with the active silanol groups takes place (Unger et al., 1979).
Selective solid phase extractors and pre-concentrators are mainly based on impregnation of the solid surface with certain donor atoms such as oxygen, nitrogen and sulfur containing compounds.
( Mahmoud 1979; Mahmoud et al., 1997). The most successful selective solid phases for soft metal ions are sulfur-containing compounds, which are widely used in different analytical fields. Amongst these sulfur-containing compounds are dithiocarbamate derivatives for selective extraction of copper(II) (Mahmoud 1999; Mahmoud 1998) and pre-concentration of various cations (Leyden et al., 1976; Moghimi et al., 2009 ;Tehrani et al., 2005) and 2- mercaptobenzothiazol-modified silica gel for on-line pre-concentration and separation of silver for atomic absorption spectrometric determinations (Moghimi et al., 2009). Ammonium hexa-hydroazepin-1-dithiocarboxylate (HMDC)-loaded on silica gel as solid phase pre-concentration column for atomic absorption spectrometry (AAS) and inductively coupled plasma atomic emission spectrometry (ICP-AES) was reported (Alexandrova et al., 1993). Mercapto-modified silica gel phase was used in pre-concentration of some trace metals from seawater (Moghimi et al., 2009). Sorption of copper(II) by some sulfur containing complexing agents loaded on various solid supports (Moghimi et al., 2011) was also reported. 2-Amino-1- cyclopentene-1-dithiocaboxylic acid (ACDA) for the extraction of silver(I), copper(II) and palladium(II) (Moghimi 2009 ), 2-[2-triethoxysilyl-ethylthio] aniline for the selective extraction and separation of palladium from other interfering metal ions (Tehrani et al., 2005) as well as thiosemicarbazide for sorption of different metal ions (Moghimi et al., 2011) and thioanilide loaded on silica gel for pre-concentration of palladium(II) from water (Tehrani et al., 2005) are also sulfur contaning silica gel phases.
Ion adsorption onto solid chelating nano polymer materials is now considered as one of the most promising techniques for selective concentration, removal and recovery of metal ions from a wide variety of sources. Among different types of polymer adsorbent, polymer fibers have attracted great interest in recent years (Tahaei et al., 2008; Moghimi , 2006). In our previous attempts, we modified SPE membrane disks with suitable compounds for selective determination of copper( Tuzen et al., 2009 ).Meanwhile, other investigators have successfully utilized these sorbents for quantitative extraction and monitoring trace amounts of copper(Tahaei et al., 2008).
Recently, CNTs have been shown to be excellent classes of sorbent materials for SPE(Moghimi 2013). Since the first application of CNTs in SPE by Cai et al that he use multi-walled carbon nanotubes (MWCNTs) (Moghimi 2013), In recent years many reports have been distributed focusing on progress of use CNTs-based SPE methods for a great variety of analytes, , inorganic ions (Moghimi et al., 2009), organometallic compounds (Kaiss et al., 2007).
In this study, we report the synthesis of this new sorbent and its application as a selective sorbent for separation. preconcentration of Cu2+ ions is done by C18 modified multi walled carbon nanotubes (MWCNTs) and Cupferron ligand and was determinate by FAAS.
Experimental
Apparatus
The concentration of the metal ion solutions was determined by using the Varian model spectra AA-240 (Mulgrara, Victoria, Australia). The pH-measurements of the metal ion and buffer solutions were carried out by an Orion 420. Infrared spectra of Multi walled carbon nanotubes (SWCNTs) were carried out from KBr by a Perkin-Elmer 1430 ratio recording spectrophotometer.
Materials and reagents
Multi walled carbon nanotubes (MWCNTs) and Schiff’s Cupferron were prepared from Merck (Darmstadt, Germany).All solutions were prepared with doubly distilled deionized water from Merck (Darmstadt, Germany). C18 powder for chromatography with diameter of about 50 µm obtained from Katayama Chemicals from Supelco. It was conditioned before use by suspending in 4 M nitric acid for 20 min, and then washed two times with water.
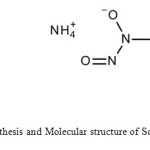 |
Schematic1: Synthesis and Molecular structure of Schiff’s Cupferron Click here to View scheme |
Preparation of Multi walled carbon nanotubes (MWCNTs) cartridge
A glass column 1.5 cm in diameter and 15 cm in length was used for the preconcentration of Copper(II). About 5 g of Multi walled carbon nanotubes (MWCNTs) cartridge was mixed with 25mL of HCl 1M to form slurry and then loaded on to the column. Cotton was placed at the bottom for allowing Multi walled carbon nanotubes (MWCNTs) cartridge to settle properly. The column was packed up to a height of 3 cm.
Procedure for preconcentration
A 50mg SDS and 30 mg Cupferron of 50mL was loaded on to the column of neutral Multi walled carbon nanotubes (MWCNTs) cartridge maintaining a flow rate of 5mL min−1. Then250 ml volume of 10 µgm Cu(II) solution was loaded on to the column. The sample solution was loaded on to the column of neutral Multi walled carbon nanotubes (MWCNTs) cartridge maintaining a flow rate of 0.5 mL min−1. The complex was adsorbed as a narrow band on the top of the column. The adsorbed complex was eluted using 10 mL of HNO3 4M at a flow rate of 0.5 mL min−1 and the concentration of Copper(II) was determined by FAAS.
Sampling
Tap water(Tehran, taken after 10 min operation of the tap),rain water(Tehran, 20January, 2014), and Sea water(taken from Caspian sea, near the Mahmoud-Abad shore) samples were analyzed(Table 2).Tap water samples used for development of the method were collected in glasses containers. Before the analysis, the organic content of the water samples was oxidized in the presence of 7% H2O2 and then concentrated nitric acid was added. These water samples were then filtered through a 0.45µm Millipore cellulose membrane to remove suspended particulate matter and stored in a refrigerator at 4 ◦C in the dark before analysis.
Results and discussion
The treatment of Multi walled carbon nanotubes (MWCNTs) (Fig.1 a,b,c,d) can lead to the Cupferron functional groups via adsorption (Fig.1 e,f,g,h,i) .The formation of MWCNTs was followed by Raman Spectroscopy. Initially, in the spectrum of MWCNTs the carbonyl vibration appears at 1580 cm-1, while there are fingerprints at 1100 cm-1 and 1300 cm-1 due to the presence of hydroxyl species at the carbon nanotubes.(Fig.2).
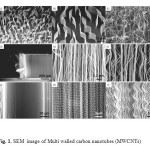 |
Figure1: SEM image of Multi walled carbon nanotubes (MWCNTs) Click here to View figure |
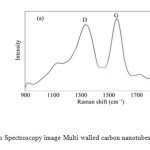 |
Figure2: Raman Spectroscopy image Multi walled carbon nanotubes (MWCNTs). Click here to View figure |
Optimization of SPE procedures
Effect of pH
The metal chelate stability constant and its chemical stability considerably influence the SPE recovery. The pH plays a very important role on metal chelate formation and following extraction. Therefore, pH was the first optimized parameter. pH of the analyte solutions was adjusted to desired values with diluted hydrochloric acid (0.1 mol L−1) and/or ammonia solution (0.1 mol L−1) The variation in recovery of Cu(II) with pH is shown in Fig.4. According to the results shown in Fig 3 up to pH 2-2.5, complete recoveries are obtained. However, at higher pH values, percentage recovery decreases. This is due to fact that in an acidic solution the protonation of Cupferron occurs and there is a weak tendency for retention between Cu (II) and C18 modified multi walled carbon nanotubes (MWCNTs) and Cupferron ligand, where as at higher values (pH>5), Cu (II) reacts with hydroxide ions to produce Cu (OH)2. Accordingly, pH 2.5 was selected for subsequent work and real sample analysis.
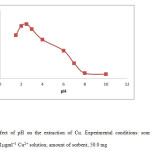 |
Figure3: Effect of pH on the extraction of Cu. Experimental conditions: source, 10 ml of 0.1µgml−1 Cu2+ solution; amount of sorbent, 50.0 mg Click here to View figure |
Effect of flow rates of sample and eluent solution
The effects of flow rates of sample solution and eluent solution on the recovery of Cu (II) was also examined between under the optimum conditions in the range of 1–10.0 mL min−1 by controlling the flow rate with peristaltic pump. The recovery of the ions were independent of flow rate in the range of 0.5–2.0 mL min−1 for eluent solution and range of 1–5.0 mL min−1 for sample solution.
Effect of sample solution volume
Another parameter studied to find the best experimental conditions is the volume of sample solution and/or analyte concentration. For this purpose, 50.0–1100.0 mL of sample solutions containing 5 ppm Cu (II) were processed according to the suggested procedure. The recovery of Cu(II) was quantitative (>96%) obtained up to a sample volume of 1000.0 mL and the adsorbed Cu(II) can be eluted with 5 mL eluent. Therefore, an enrichment factor of 200 was achieved by this technique. Finally In our suggested procedure, a sample volume of 50.0 mL was chosen for preconcentration method.
Effect of amount of sorbent
To achieve a high extraction recovery, different amounts of multi walled carbon nanotubes (MWCNTs) and Cupferron ligand ranging from 50 to 300 mg were applied to extract the target compounds from the sample solutions. The results are shown in Fig 4 from which it can be seen that the extraction recovery achieved by 100 mg, but almost the same as obtained with 300 mg or more than of the adsorbent. Based on the above results, 100 mg of multi walled carbon nanotubes (MWCNTs) and Cupferron ligand was selected for the following experiments.
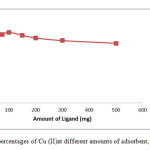 |
Figure4: Removal percentages of Cu (II)at different amounts of adsorbent, of Cu (II), pH= 2.5. Click here to View figure |
Eluent type and its volume
Another important factors which affect the percent of recovery are the type, volume, and concentration of the eluent solution used for the removal of metal ions from the sorbent. For this purpose, various type of eluents were examined according to the suggested procedure. 4.0 mol L−1 HNO3 was found to be adequate for quantitative elution (≥95%). The results show the best quantitative recovery is 5.0 mL of 4.0 mol L−1HNO3 . As a result 5.0 mL of 4.0 mol L−1 HNO3 was selected in the subsequent preconcentration method.
Reusability of Column
The stability and potential regeneration of the column were studied. After every time extraction, the column was washed with 10 mL of MeOH and 10mL of deionized water. Thus, the column was available for a next extraction immediately at least 50 adsorption elution cycles without significant decrease in the recovery of Cu (II) ions.
Effect of foreign ions
The influence of common foreign ions on the adsorption of Cu (II) on C18 modified multi walled carbon nanotubes (MWCNTs) and Cupferron ligand were studied. In these work, 50.0 ml solutions containing 5 ppm of Cu and various amounts of interfering ions were treated according to the suggested procedure. The tolerance level was defined as the maximum concentration of the foreign ion causing a change in the analytical signal no higher than 5%, when compared with the signal of 5 ppm Cu alone. The results, listed in Table 1, demonstration that the presence of major cations and anions in natural water has no important influence on the adsorption of Cu (II) ions under the designated conditions.
Table 1. Separation of Cu from binary mixtures a
|
%Recovery of Cu2+ ion |
Amounts taken(mg) |
added |
Diverse ion |
|
97.6(0.5)b |
100 |
NaNO3 |
|
|
98.1(0.3) |
100 |
KCl |
|
|
99.7(0.9) |
100 |
Ca(NO3)2 |
Ca2+ |
|
99.2(1.3) |
100 |
Mg (NO3)2 |
Mg2+ |
|
98.8(1.1) |
80 |
Fe (NO3)3 |
Fe3+ |
|
98.9(0.7) |
80 |
Cd (NO3)2 |
Cd2+ |
|
99.1(1.3) |
80 |
Cr (NO3)3 |
Cr3+ |
|
97.6(0.6) |
50 |
Co (NO3)2 |
Co2+ |
|
99.5(1.1) |
50 |
Ni(NO3)2 |
Ni2+ |
|
98.3(0.8) |
50 |
NaCl |
|
|
97.4(0.5) |
20 |
NaBr |
|
|
97.3(0.9) |
20 |
NaF |
|
|
98.7(1.2) |
20 |
MnCl2 |
Mn2+ |
|
99.7(0.8) |
20 |
ZnCl2 |
Zn2+ |
aInitial samples contained 10µg Cu 2+ and different amounts of various ions in 100 mL water.
bValues in parentheses are RSDs based on five individual replicate analysis.
Analytical figures of merits
Under optimized conditions, a calibration curve for Cu (II) was found by preconcentrating a series of Cu (II) standards according to the procedure mentioned. The curve was linear from 1.0 mg/l to 7.0 mg/l for Cu.
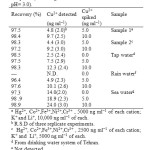 |
Table2: Recovery of Cu(II) added to 100mL of different water samples (contaning 0.1M buffer acetic acid / acetate at pH= 3.0). Click here to View table |
Determination of Copper in real water samples
Three type of water samples (information described in section 2.6 sampling) were used for the determination of Copper. The analytical results, are given in Table 2. The percent of recoveries for the addition of different concentrations of Cu (II) to water samples were 97 and 98.9%.These satisfactory percent of recoveries indicate no significant effects from the matrix composition of the real water samples.
Conclusion
In conclusion, the proposed SPE possesses the following advantages: the technique is rapid when compared with the previously reported procedures for the separation and determination of Copper, the time taken for the separation and determination of Copper in a 500 mL sample is at the most 30 min. Furthermore, it is a simple, highly sensitive, selective and reproducible method for the separation of Cu2+ and in this work the recovery yields obtained with C18 modified multi walled carbon nanotubes (MWCNTs) and Cupferron ligand were about 95-100% then it show C18 and its derivation e.g. in this work C18 modified multi walled carbon nanotubes (MWCNTs) and Cupferron ligand is full of potential for use as a adsorbent in the extraction method like SPE and SPME .consequently it can be applied to the preconcentration and determination of Copper and the large number of heavy metal that are dangers for human from real samples.
Reference
- Alexandrova A., Arpadjan S., Column solid phase extraction as preconcentration method for trace element determination in oxalic acid by atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry Analyst 1993, 118, 1309-1314.
- Boudreau S.P., Cooper W.T., Analysis of thermally and chemically modified silica gels by heterogeneous gas-solid chromatography and infrared spectroscopy Anal. Chem. 1989, 61: 41-47.
- Bruening M.L., Mitchell D.M., Bradshaw J.S., Izatt R.M., Bruening R.L., Effect of organic solvent and anion type on cation binding constants with silica gel bound acrocycles and their use in designing selective concentrator columns, Anal. Chem. 1991; 63: 21-27.
- Caroli C., Alimanti A., Petrucci F., Horvath Zs., Determination of trace elements in analytical-reagent grade sodium salts by atomic absorption spectrometry and inductively coupled plasma atomic emission spectrometry after preconcentration by column solid phase extraction Anal. Chim. Acta. 1991, 248, 241-245.
- Choi,Y.S.;Choi,H.S., High Functional Inorganic Polymers Containing Main Group 13 16 Elements in the Polymer Backbone Chain Bull. Korean Chem. Soc.2003, 24: 222-228.
- Geim A. K., Novoselov K. S., The rise of grapheme Nat. Mater., 2007, 6, 183-190.
- Gennaro M.C., Baiocchi C., Campi E., Mentasti E., Aruga R., Preparation and characterization of iminodiacetic acid—cellulose filters for concentration of trace metal cations Anal. Chim. Acta 1983, 151: 339-344.
- Jones J.S., Harrington D.E., Leone B.A., Bramdstedt W.R.,Application of optical emission source developments in metallurgical., Atom. Spectrosc. 1983, 4: 49-54.
- Kvitek R.J., Evans J.F., Carr P.W. , Diamine/Silane-Modified controlled pore glass: The covalent attachment reaction from aqueous solution and the mechanism of reaction of bound diamine with copper Anal. Chim. Acta 1982, 144: 93-97.
- Kaiss .R.,Waleed .F and Mohammed A., Synthesis and Photolysis of Some Transition Metal Complexes of Schiff Base Ligand Derived From Ethylene Diamine and Salicylicaldehyde. J.of Al-Anbar university for pure science 2007, 1(1).
- Leyden D.E., Luttrell G.H., Nonidez W.K., Werho D.B., ESCA-studies on activated silicagel surfaces preconcentrating heavy metal ions Anal. Chem. 1976, 48, 67-74.
- Leyden D.E., Luttrell G.H., Preconcentration of certain anions using reagents immobilized via silylation Anal. Chim. 1975, 47:1612-1619.
- Mahmoud M.E., Silica gel-immobilized Eriochrome black-T as a potential solid phase extractor for zinc (II) and magnesium (II) from calcium (II)Talanta 1997, 45: 309-314.
- Mahmoud M.E., Soliman E.M., Study of the selective extraction of iron (III) by silica-immobilized 5-formyl-3-arylazo-salicylic acid derivatives Talanta 1997, 44, 1063-1069.
- Mahmoud M.E., in: Proceeding of the 25th FACSS Conference, Austin, TX, USA, 11–15 October, 1998.
- Mahmoud M.E., Selective solid phase extraction of copper(II) by silica gel-immobilized-dithiocarbamate derivatives Anal. Chim. Acta 1999, 398, 297-302.
- Moghimi A., Abedin A.R. Shahriar Ghammamy S. , Ghiasi R., Solid phase extraction of Cd (II) using mesoporous organosilicas and determination by FAAS African Journal of Pure and Applied Chemistry 2009, 3(3): 051-059.
- Moghimi A., Preconcentration and Determination of Fe(III) Using Octadecyl SilicaMembrane Disks and Flame Atomic Absorption Spectrometry Oriental Journal of Chemistry 2006, 22(3): 527-535.
- Moghimi A., Shahriar Ghammamy S. , Ghiasi R., A study on the solid phase extraction of CO(II)-IIDE chelate with C18 disk and its application to the determination of trace cobalt African Journal of Pure and Applied Chemistry 2011, 5(6): 14.
- Moghimi A, Ghiasi R, Abedin AR, Ghammamy S., Solid phase extraction of Cd(II) using mesoporous organosilicas and determination by FAAS. Afr. J. Pure Appl. Chem. 2009, 3(3):051-059.
- Moghimi A., Separation of copper(II) paraffin-embedded tissues from liver loggerhead turtles specimens by organic solution processable functionalized-nano graphene prior to determination by flame atomic absorption spectrometry (FAAS). Afr.J. Pure Appl. Chem. 2013, 7(2):79-90.
- Nambiar D.C., Patil N.N., Shinde V.M., Liquid-liquid extraction of copper (II) with triphenylphosphine sulphide: Application to medicinal and environmental samples Fresenius J. Anal. Chem. 1998, 360: 205-211.
- Shamsipur, M.; Shokrollahi, A.; Sharghi, H.; Eskandari, M. M., Solid phase extraction and determination of sub-ppb levels of hazardous Hg2+ ions J.Hazard. Mater, 2005, 117-122.
- Shuai Wang B., Jon Chia P., Chua L., Hong Zhao L., Qi Png R., Sivaramakrishnan S., Wee S., Ho H., Band-like Transport in Surface-Functionalized Highly Solution-Processable Graphene Nanosheets Adv. Mater. 2008, 3440–3446.
- Tahaei P., Abdouss M., Edrissi M., Shoushtari A. M., Zargaran M., Preparation of chelating fibrous polymer by different diamines and study on their physical and chemical properties Mat.-wiss. u. Werkstofftech. 2008, 39: 839–844.
- Tehrani M.S., Moghimi A., Waqif Husain S., Solid Phase Extraction of Cr (III) from Natural Water by Modified Nano Polyacrylonitrile Fiber, Material Science Research India 2005, 3(2): 135-142.
- Tuzen M., Karaman I., Citak D., Soylak M., Mercury(II) and Methyl Mercury Speciation on Streptococcus Pyogenes Loaded Dowex Optipore SD-2, Journal of Hazardous Materials, 2009, 169: 345-350.
- Unger K., Comparison of an ordered mesoporous aluminosilicate, silica, alumina, titania and zirconia in normal-phase high-performance liquid chromatography Porous Silica, Elsevier, Amsterdam, 1979.
- Wood M., Wang H.K., Biosorption of cadmium by CO2-fixing microalga Scenedesmus obliquus CNW-N Environ.Sci.Technol.1983, 17, 582-588.

This work is licensed under a Creative Commons Attribution 4.0 International License.









