PhotoCatalytic Degradation of Methyl Blue Dye and Enhanced Antibacterial Properties of Treated Wool with ZrCL4 and Dyeing with Madder
Arash Almasian1, Laleh Maleknia2,*, Abosaid Rashidi3
1Young Researchers Club, Islamic Azad University, Tehran South Branch, Tehran, Iran, 2*Department of Textile Engineering, Islamic Azad University, Tehran South Branch, Tehran, Iran,
3Department of Textile Engineering, Azad University, Science and research Branch, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/310117
Article Received on :
Article Accepted on :
Article Published : 08 Feb 2015
This research aims to create the photo catalytic degradation of methyl blue dye and enhanced antibacterial properties on wool fabric using ZrCl4 particles. Wool fabrics were treated with different concentrations of zirconium salt in water solution including %1, %3, %6 and 9% o.w.f. and the dyeing process was carried out on the fabrics in the states before, simultaneously and after the mordanting with madder.Formic acid was used to generate acidic pH.The photo catalytic properties of the fabrics were examined through evaluating the color removal from the stained fabric with Methylene Blue. The antibacterial properties were determined by reduction growth of a Gram-negative bacterium E. coli and a Gram-positive bacterium Staphylococcus aureus. Furthermore, the FTIR spectrum of some samples was analyzed. The SEM pictures and EDX spectrums of some samples were also reported.
KEYWORDS:antibacterial; photo catalytic degradation; zirconium; wool fabric
Download this article as:| Copy the following to cite this article: Almasian A, Maleknia L, Rashidi A. PhotoCatalytic Degradation of Methyl Blue Dye and Enhanced Antibacterial Properties of Treated Wool with ZrCL4 and Dyeing with Madder. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Almasian A, Maleknia L, Rashidi A. PhotoCatalytic Degradation of Methyl Blue Dye and Enhanced Antibacterial Properties of Treated Wool with ZrCL4 and Dyeing with Madder. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7045 |
Introduction
Natural dyes or colorants derived from flora and fauna are believed to be safe because of their non-toxic, non-carcinogenic and biodegradable nature. The present trend throughout the world is shifting towards the use of ecofriendly and biodegradable commodities. This leads to increasing demand for natural dyes [1]. Madder is widely distributed in southern and southeastern Europe, the Mediterranean area and in Asia. It is a main source of natural dye producing a variety of anthraquinone pigments in its roots and rhizomes [2–4]. Natural dye madder is not able to create a strong bond with the wool fiber. Therefore, it should be used as a substance for helping create a strong bond between dye and fiber. Mordant is the substance of helping, which the two reasons are important: 1. creates a link with the dye and affect on the color fastness, however, the mordant should be optimized; 2. the mordant effect on the shade therefore wide range of mordant can be achieved wide shads. Nearly all previous research is focused on the enzyme treated and inorganic mordants (AL,Cu, Zn, Ne…) on the wool.
Extracts obtained from many plants have recently gained popularity and scientific interest. Many plants have been used for different purposes, such as food, drugs and perfumery [5]. Researchers have been interested in biologically active compounds isolated from plant species for the elimination of pathogenic microorganisms because of the resistance that microorganisms have built against antibiotics [6].
The genus Rubiais represented in the Turkish flora by five species, one of which is endemic. R. tinctorumgrows in the west, south and south east parts of Europe[7]. Some members of this genus are of economicimportance. Common Madder (R. tinctorumL.)produces anthraquinone pigments in its roots, one of them being alizarin (1,2dihydroxyanthraquinone)which has been used for dyeing textiles since 2000 BC[8]. The underground parts of R.tinctorumhave been used as a natural dye in Turkey[9].
This study was aimed to determine the antimicrobial activity of madder against various microorganisms and the self-cleaning properties of the wool fabrics.
Experiments
Materials
The wool fabric with the weight 1688.55 g/m2, width 140 cm was used from Iran Merinos Co. The anionic detergent was used for scouring the wool. The mordant used was zirconium chloride (IV) (ZrCl4). It was produced by Merck. Also, formic acid from Merck was applied for dyeing process. Powder of Iranian madder roots was used for dyeing of wool fabrics.
Method
The mordanting treatments were carried out before, simultaneously and after the dyeing. Zirconium salt concentrations of %1, %3, %6 and 9% o.w.f. were used and the liquor ratio was 50:1. Following the mordanting treatment, the fabrics were maintained for 1h at a temperature of 85◦C. Dye solutions were prepared 24 h prior to dyeing by adding madder powder to water (50%o.w.f., liquor ratio 50:1). The dyeing process was started at 40 ◦C and the temperature was increased to 85 ◦C over 20 min and then held at that temperature for 1 h; finally, the fabrics were rinsed. The acidic pH was maintained using formic acid.
Analytical Methods
Fourier-Transform Infrared Spectroscopy (FTIR)
The chemical compositions of the fabrics were examined by the FTIR spectroscopy [Bomem-MB 100 Series (Hartmann and Broun)rsqb].
Photo Catalytic Degradationof Methyl Blue on Wool Samples
A 5 μl of 5% MB was introduced onto the wool sample and the fabric was irradiated under UV lamp at ambient temperature for 24 and 48h. The distance between the fabric sample and the lamp was 40 cm. The fabrics’ photo catalytic degradation were determined using a Gretagmacbeth COLOREYE 7000A spectrophotometer integrated with an IBM-PC. CIELAB color coordinates (L*, a*, b*, C*, h˚) and color differences (ΔE) of sample were calculated for 10˚ observer and D65 illuminant according to equation 1.
ΔE=[(Δ a*)2+(Δ b*)2+( Δ L*)2]1/2 (1)
Evaluation of antibacterial efficiency
The antibacterial activity of the treated wool fabric was tested against two bacteria, Staphylococcus aureus (S. aureus), American Type Culture Collection No.6538, as a gram positive bacterium and Escherichia coli, American Type Culture Collection No.11303, as a gram negative bacterium. To this end, some colonies of each bacterium were suspended in a physiologic saline solution (NaCl 0.9% in distilled water at pH 6.5) with concentration of 0.5 Mc Farland. The vials of bacterial suspensions were then incubated with agitation at 37ºC ± 2ºC, 220 rpm for 2 hours. A homogenous suspension of bacteria was prepared and serial dilution was then done in 5 Steps (dilution of 1:100000). A concentration of about 1.5-2Í10³ CFU/ml was applied for the antibacterial testing. The bacteriological culture tubes (ie, 125 Í17 mm glass tubes), containing one piece of the treated wool fabric (10 mmÍ10mm) were sterilized by an autoclave device in moisturized heat (121º C, 15 Ib) for 15-20 minutes. An aliquot of 1cc bacterial suspension and 2cc of TSB (Tryptic Soy Broth) was then added to every tube and 3 ml was detected in each tube. To ensure that any decrease in bacterial count was due to the exposure to the wool fabrics, one control of the saline solution with TSB, and one control of aliquot with the untreated fabrics including the tubes containing the treated wool fabrics with the bacterial suspensions and the control tubes were incubated at 37ºC for 24h. The samples of 10 µl from each tube were then taken and counted according to the pour plate method. Thus, samples were mixed with melted agar (that decreases the temperature down to 45ºC) and poured. The plates incubated at 37ºC for 24h and the colonies of each plate were counted by a colony counted device to determine the bacteria reduction of the suspensions. The results of the numbers before and after the treatment with the wool fabrics were used to determine the bactericidal effect and the percentage of reduction of bacteria was calculated using the following equation:
Reduction rate (%) = 100 (A-B)/A (2)
Where R is the bacterial reduction ratio, A is the number of bacterial colonies from the untreated fabrics and B is the number of the bacterial colonies from the treated fabrics.
Results and Discussion
Structural Information by FTIR Spectra
The infrared spectra of untreated wool as well as the sample mordanted with zirconium salt are shown in Figure 1. The N-H stretching and bending vibrations in wool are usually appeared at 3100-3500 cm-1 and 1550-1640 cm-1 respectively, depending on the type of amide (primary and secondary), chemical environment (solid and liquid) and intra- or inter-molecular hydrogen bonds. The C=O stretching vibration band appears in the normal region between 1630 and 1670 cm-1which usually overlaps with N-H bending [10, 11].
Figure 1a present N-H stretching of a secondary amide at 3251 cm-1 as expected for wool. The bands at 1634 cm-1 are also characteristic of N-H stretching vibration which overlaps with C=O vibration. The weak C-H stretching, CH2 out of plane bending, C-O-C asymmetric stretching and S-O-S (cystine monoxide) vibrations in wool appear at 2919, 835, 1232 and 1072 cm-1, respectively. Other weak bands appeared at 3759 and 3682 cm-1 are attributable to O-H stretching vibrations in free primary and secondary alcohols [11].
Figure 1b indicates formic acid treated wool / 9% zirconium salt, the intensity of bands at 3811 and 3852 cm-1were increased and new band at 1701 cm-1was appeared. These changes are due to interaction OH groups of wool and interaction between carboxylic wool and formic acid cations. The band at 1391 cm-1 was disappeared due to CH stretching and bending vibration after mordanted. The intensity of the bands at 1065 and 502 cm-1 were increased, that are due to interaction between formic acid anions and amino groups of wool and change of OCN conformation in amide groups, respectively.
As can be seen in the Figure 1c, dyeing wool structure was the same as untreated wool. The intensity of band at 3851 cm-1was increased. Its change is due to dye absorbent and increment of OH bands of anthraquinone in wool.
As can be seen in the Figure 1d, decrement in the intensity of band at 3516 cm-1 can be expressed interaction between OH anthraquinone of madder on one hand and wool and mordant cations on the other hand was decreased free OH groups in wool.
 |
Figure1: FTIR spectra a) untreated wool, b) 9% zirconium salt treated wool, c) madder dyeing wool, d) 9% zirconium salt / madder wool Click here to View figure |
Microscopic Characterization
The SEM images of untreated wool fiber and the samples treated with formic acid and different concentrations of zirconium salt are shown in Figures 2a-g. It can be observed that the scales have damaged in dyeing with madder and formic acid treated fiber. The zirconia particles can be observed on samples of formic acid treated. It is evident that increment in zirconium salt concentration increased the aggregated particles on the surface of the fiber. In the sample of formic acid in Figure (a) can be observed presence of particles which the tip of the scales is probably isolated by formic acid.
 |
Figure2: SEM images (a) dyeing with madder wool fiber, (b) wool fibers dyeing with madder and 1%zirconium salt in before mordant method (c) wool fibers dyeing with madder and 9%zirconium salt in before mordant method (d) wool fibers dyeing with madder and 1%zirconium salt in simultaneously mordant method (e) wool fibers dyeing with madder and 9%zirconium salt in simultaneously mordant method (f) wool fibers dyeing with madder and 1%zirconium salt in after mordant method (g) wool fibers dyeing with madder and 9%zirconium salt in after mordant method.
|
Figure 3a-g and Table 1 show the presence of chemical elements on the surface of untreated fiber as well as on the surface of wool dyeing with madder and zirconium salt as investigated by EDX analysis. In these patterns, Au peaks clearly show that gold is successfully coated on surfaces of all fibers; and there is no Zr present on untreated wool. EDX analysis of cross-linked wool illustrates more efficient interactions between wool surface and zirconium salt leading to presence of Zr at this sample´s surface. The increment of zirconium salt concentration increased presence of Zr and in after mordant method be seen Zr more due to zirconium salt is a after- treatment method.
Table 1: Energy dispersive X-ray analysis
|
Formic acid
|
Quantity of zirconium salt (O.W.F%) |
method |
||
|
Au(%) |
Zr(%) |
|||
|
91.66 |
8.34 |
1 |
before mordant |
|
|
89.97 |
10.03 |
9 |
||
|
91.91 |
8.09 |
1 |
simultaneously mordant |
|
|
89.67 |
10.33 |
9 |
||
|
89.66 |
10.34 |
1 |
after mordant |
|
|
87.21 |
12.79 |
9 |
||
 |
Figure3: Energy dispersive X-ray analysis (a) dyeing with madder wool fiber, (b) wool fibers dyeing with madder and 1%zirconium salt in before mordant method (c) wool fibers dyeing with madder and 9%zirconium salt in before mordant method (d) wool fibers dyeing with madder and 1%zirconium salt in simultaneously mordant method (e) wool fibers dyeing with madder and 9%zirconium salt in simultaneously mordant method (f) wool fibers dyeing with madder and 1%zirconium salt in after mordant method (g) wool fibers dyeing with madder and 9%zirconium salt in after mordant method. Click here to View figure |
Several factors affect on the ability of zirconium salt to aggregate on the surface of textiles including size, mobility, end-group functionalities, relative composition and molecular architecture. It seems that interactions between wool and zirconium salt are strong enough to enable deposition of zirconium salt particles on the wool surface as a result of their high surface area.
Photo Catalytic Degradationof Methyl Blue on Wool Samples
The surface of ZrCl4 is struck by UV light (hv) resulting to excitation of valence band (vb) electrons and jumping to the conduction band (cb). Highly reactive electron (e–) and hole pairs (h+) are produced after this step and the negative charge is increased in the conduction band (e–cb). Furthermore, the reactive electrons interact with dissolved O2 molecules to form super oxide radical anions which results to the formation of HO2.radicals. In other words, the positive holes react with water molecules to form .OH radicals. Figure 4 shows the mechanism for Photo-reduction phenomenon of zirconium salt under UV irradiation [13-15].
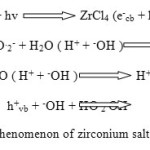 |
Figure4: Photo-reduction phenomenon of zirconium salt under UV irradiation Click here to View figure |
On the other hand, UV irradiation of wool causes scission of the main functional groups including carboxyl and hydroxyl bonds as well as cystine linkages which may result in formation of alkyl, alkoxy and alkyl peroxy radicals in wool. This photochemical deterioration results in both yellowing and changes to the mechanical properties of fiber [ 13-21]. Figure 5 illustrates various free radicals formed in wool by UV light.
![Fig5: Free radicals produced in wool by UV irradiation[13-21]](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_Phot_Aras_Fig5-150x150.jpg) |
Figure5: Free radicals produced in wool by UV irradiation[13-21] Click here to View figure |
Table 2, Figure 6 and 7 present the color co-ordinates, the reflectance curves and images for wool samples dyeing with madder / 9%zirconium salt and mordanted of 9% zirconium salt , stained by methyl blue before irradiation and after 24 and 48h UV irradiation.
The color values were evaluated in CIELAB color space, the three axes being L*, a*, and b*. The L* is the color coordinate which represents the lightness of samples and can be measured independently of color hue. Any decrease in the lightness of samples could be concluded as the lower reflectance of textiles. The a* stands for the horizontal red–green color axis. The b* represents the vertical yellow–blue axis. The C* represents the brightness or dullness of the samples. Any increase in the C* of samples could be concluded as greater brightness of the composite. The hue angle (h°) stands for hue, which is the actual color recognized by the human eye and identified as orange, yellow, beige, brown, pink, or any of the other colors visible to humans. It is expressed in degrees, with 0° being a location on the +a* axis, continuing to 90° for the +b* axis, 180° for -a*, 270° for -b*, and back to 360° = 0°. Differences in color coordinate of samples before and after UV irradiation are defined by ΔE.
As it can be seen in Table 2, wool samples showed higher L* values and lower brightness after UV irradiation. Both Samples stained showed a great increase in redness after exposing to UV light.
Table 2: The color co-ordinates for stained wool samples dyeing with madder / 9%zirconium salt and mordanted of 9%zirconium salt before irradiation and after 24 and 48h UV irradiation
| <> | dyeing with madder / 9%zirconium salt | mordanted of 9%zirconium salt | ||||||||||
| Methyl blue stained Sample |
L* |
a* |
b* |
C* |
h˚ |
ΔE | L* | a* | b* |
C* |
h˚ |
ΔE |
|
Before UV irradiation |
37.50 |
-4.38 |
11.24 |
12.06 |
111.30 |
– |
65.45 |
-22.76 |
-11.21 |
25.37 |
206.22 |
– |
|
After UV irradiation for 24 h |
44.68 |
10.03 |
19.88 |
22.27 |
63.22 |
18.28 |
71.47 |
-7.23 |
1.01 |
7.30 |
172.05 |
20.65 |
|
After UV irradiation for 48 h |
48.70 |
13.50 |
22.70 |
26.23 |
59.91 |
22.96 |
75.96 |
-4.48 |
6.07 |
7.54 |
126.44 |
27.75 |
Results obtained from Figure 6 indicated that the reflectance values for UV irradiated samples were higher as compared to those unexposed to UV lamp. This can be due to decomposition of dye stains and photo-catalytic activity zirconia particles embedded in coating. The migrated electrons and holes of zirconia produce OH radicals which are able to degrade dye stains.
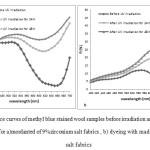 |
Figure6: Reflectance curves of methyl blue stained wool samples before irradiation and after 24 and 48 h UV irradiation for a)mordanted of 9%zirconium salt fabrics , b) dyeing with madder / 9%zirconium salt fabrics Click here to View figure |
 |
Figure7: Discoloration of methylblue stain on the wool sample before irradiation and after 24 and 48h UV irradiation for a) dyeing with madder / 9%zirconium salt fabrics, b)mordanted of 9%zirconium salt fabrics Click here to View figure |
Figure 8 shows the mechanism for photocatalysis of methyl blue by ZrCl4 under UV irradiation. At the initial step, the functional groups of C-S+=C is degraded to sulfoxide groups which subsequently may undergo a second attack by an OH radicals producing the sulfone and causing the definitive dissociation of two benzenic rings. The sulfone can be attacked itself by a third OH radical for giving a sulfonic acid which finally release the SO42− ions.
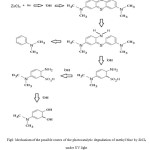 |
Figure8: Mechanism of the possible routes of the photocatalytic degradation of methyl blue by ZrCl4 under UV light Click here to View figure |
Antibacterial Properties of Wool Samples
The antibacterial effect of natural dye dependsphenelic, polyphenol, phelaonol, tanon, komarin, poly peptid groups in antibacterial compounds.The antibacterial effect of madder depends to very antibacterial compounds in plant and metabolic toxins. Also, methanol extract of madder hasantibacterial effect that maybe is from komarin groups. Figure 9 and Table 3 depicts the colonies of S. aureus and E. coli corresponding untreated wool and the samples treated.The results showed that madder and zirconium salt have a positive effect on antibacterial properties of the treated textile samples.The reduction rates for both Gram-positive and Gram-negative bacteria were good.
Table 3: Antibacterial test result against E. coli and Staphylococcus aureus A) untreated wool B) mordanted with 9% zirconium salt wool C) dyeing with madder wool D) dyeing with madder and 9% zirconium salt wool
|
|
Staphylococcus aureus |
E.Coli |
||
|
sample |
Number of colony |
Reduction of bacteria (%) |
Number of colony |
Reduction of bacteria (%) |
|
A |
∞ |
0% |
∞ |
0% |
|
B |
360 |
43.75% |
400 |
32.10% |
|
C |
7 |
98.90% |
21 |
96.44% |
|
D |
3 |
99.53% |
17 |
97.11% |
 |
Figure9: Antibacterial test result against E. coli (right) and Staphylococcus aureus (left) A) untreated wool B) mordanted with 9% zirconium salt wool C) dyeing with madder wool D) dyeing with madder and 9% zirconium salt wool Click here to View figure |
The results show that madder dye adds an adhesive ability to microorganisms.They can be used in the removal of a diverse range of biological contaminants including bacteria, virusesand natural organic matters. This is due to madder antibacterial compounds which impinge the bacterial cell surface, disrupting the intracellular metabolic pathways. Subsequently, the internal contents are released due to the cell rupture caused by the oxidative stress
Conclusion
This study aims to clarify the action of bio catalysts and antibacteriali in enhancing photocatalytic action of ZrCl4 treated textiles. Wool fabrics were mordanted with 1, 3, 6 and 9% zirconium salt solutions and dyed with madder. The FTIR spectra showed which seems to increase the interfacial interactions and bonding with the amine or hydroxyl end groups of wool chains by interactions and changes of conformation. Results obtained from color measurement indicated that the reflectance values for UV irradiated samples were higher as compared to those unexposed to UV lamp. This can be due to decomposition of dye stain and photo-catalytic activity of zirconium particles embedded in wool. It was also indicated that wool fabrics have a good antibacterial for both Gram-positive and Gram-negative bacteria.
References
- Farizadeh K., Yazdanshenas M.E., Montazer M., MalekR.M.A., Rashidi A.TEXT RES J,2010; 80:847–855.
- Banyai, P., Kuzovklina, I.N., Kursinszki, L., and Szoke, E.Chromatographia Supplement,2006; 63: 66–72 .
- Derksen, G. C. H., Naayer, M., Beek, T. A. V., Capelle, A., Haaksman, I. K., Doren, H. A.V., and Groot, E. D.Phytochem. Anal.,2003; 14: 137–144.
- Derksen, G. C. H., Lelyveld, G. P., Beek, T. A. V., Capelle, A., and Groot, E. D., Phytochem. Anal., 2004;15: 397–406.
- Heath HB. 1981. Source Book of Flavours. AVI: Westport, 890.
- Essawi T, Srour M. J Ethnopharmaco 2000;l70: 343–349.
- Davis PH. Flora of Turkey and the East Aegean Islands. Edinburgh University Press: Edinburgh. 1988.
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Test, 6th edn.Approved Standard M2-A6, USA. National Committee for Clinical Laboratory Standards: Wayne, USA. 1997.
- Baytop Therapy with Medicinal Plants in Turkey. Publication 3255. Istanbul University: Istanbul. T. 1984.
- M. Parvinzadeh, R. Assefipour, A. Kiumarsi, Polym. Degrad. Stabil.2009; 94: 1197-1205.
- Parvinzadeh M.,Enzym. Microb. Technol. 2007;40: 1719.
- Montazer M., Taghavi F. A., Toliyat T., BameniMoghadam M., J ApplPolymSci, 2007;106: 1614–1621.
- Kohno Y., Tanaka T., Funabiki T., Yoshida S., Faraday Trans., J. Chem. Soc., 1998; 94: 1875-1880.
- Wu C., Zhao X., Ren Y., Yue Y., Hua W., Cao Y., Tang Y., Gao Z., MolCatal A: Chemical, 2005;229: 233–239.
- Anpo M., Moon S.C., Res. Chem. Intermed., 1999;25: 1-12.
- Parvinzadeh M., Eslami S., Chemical Intermediates. 2011 Article in Press.
- Hakansson E., Kaynak A., Lin T., Nahavandi S., Jones T., Hu E., Synthetic Metals,2004; 144: 21-28.
- Mičušík M., Nedelčev T., Omastová M., Krupa I., Olejníková K., Fedorko P., Chehimi M. M., Synthetic Metals,2007; 157: 914-923.
- Kohno Y., Tanaka T., Funabiki T., Yoshida S., Faraday Trans., J. Chem. Soc., 1998;94: 1875-1880.
- Wu C., Zhao X., Ren Y., Yue Y., Hua W., Cao Y., Tang Y., Gao Z., MolCatal A: Chemical,2005; 229 : 233–239.
- Anpo M., Moon S.C., Res. Chem. Intermed., 1999;25: 1-12.

This work is licensed under a Creative Commons Attribution 4.0 International License.









