Methane Sulphonic Acid is Green Catalyst in Organic Synthesis
Pramod Kulkarni
Hutatma Rajguru Mahavidyalaya Rajgurunagar Pune 410505 MS India
DOI : http://dx.doi.org/10.13005/ojc/310154
Article Received on :
Article Accepted on :
Article Published : 23 Feb 2015
Methane sulphonic acid is an alkanesulphonic acid and its chemical formula is CH3SO3H. MSA is a strong acid having pKa= 1.9 and completely ionized in 0.1 M in an aqueous solution and has small affinity to oxidize organic compounds, less corrosive and toxic than other mineral acids. MSA is also biodegradable and not evolve toxic gases. Therefore MSA is considered as green acid. Therefore its use in organic synthesis attracts many chemists to use in organic synthesis. In this review we described the MSA catalyzed organic transformation.
KEYWORDS:Methane sulphonic acid; green; catalyst; organic reactions
Download this article as:| Copy the following to cite this article: Kulkarni P. Methane Sulphonic Acid is Green Catalyst in Organic Synthesis. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Kulkarni P. Methane Sulphonic Acid is Green Catalyst in Organic Synthesis. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7368 |
Introduction
Physical properties of Methane Sulphoic Acid
Methane sulphonic acid is a clear colourless liquid available as a 70% solution in water and anhydrous form. The structure of methane sulphonic acid lends itself to many catalytic reactions, due to its high acid strength (pKa= -1.9) and low molecular weight (96.0 g/mol).
Methane Sulphonic Acid is Green Acid Catalysts
- It is easy to handle methane sulphonic acid as liquid and can be recyclized.
- It has low LD50 and biodegradable forming sulphate and CO2.
- Methane sulphonic acid is considered to be natural product and is part of the natural sulphur.
- It is less corrosive and toxic than other mineral acids.
Due to these properties methane sulphonic acid making an environmentally benign material [1].
Applications of Methane Sulphonic Acid as Catalyst in Organic Synthesis
1. H. Naeimi et. al used methane sulphonic acid as catalyst for regioselective route for Direct Ortho-Acylation of Phenols and Naphthols under Microwave condition [2].
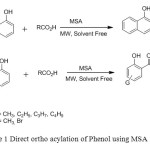 |
Figure1: Direct ortho acylation of Phenol using MSA Click here to View figure |
2. M. Heravi et. al use MSA as catalyst for One-pot synthesis of 2-Amino-4 H-chromenes [3].
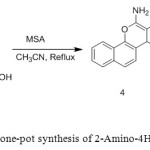 |
Figure2: MSA catalyzed one-pot synthesis of 2-Amino-4H-chromenes Click here to View figure |
3. V. Jhansi Rani et. al use MSA for synthesis of 1-amidoalkyl-2-naphthols [4]
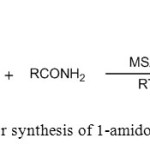 |
Figure3: Use of MSA for synthesis of 1-amidoalkyl-2-naphthols Click here to View figure |
4. P. Kulkarni et. al use MSA for synthesis of flavanone [5].
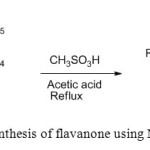 |
Figure4: Synthesis of flavanone using MSA Click here to View figure |
5. H. Sharghi et. al use MSA with Al2O3 for synthesis of monoesterification of diols [6].
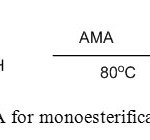 |
Figure5: Use of MSA for monoesterification of diols Click here to View figure |
6. Methanesulphonic acid in combination with morpholine used as catalyst for the preparation of ylidenemalononitriles via Knoevenagel condensation of ketones with malononitrile under solvent free condition [7].
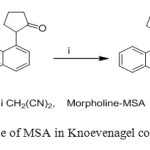 |
Figure6: Use of MSA in Knoevenagel condensation Click here to View figure |
7. Phosphorus pentoxide-Methanesulphonic acid (Eaton’s reagent) used catalyst for an expeditious, one-pot method for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent free condition[8].
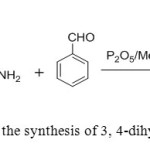 |
Figure7: Use of P2O5/ MSA in the synthesis of 3, 4-dihydropyrimidin-2(1H)-ones
|
8. Hydrofluoric acid was used as catalyst for synthesis of paracetamol from phenyl acetate but tis acid is toxic and hazardous. A. Commarieua et. al uses methane sulphonic acid as a catalyst for this reaction to give para-hydroxyacetophenone with high conversion and selectivity (up to 92% of para-isomer and 8% of ortho-isomer at 100 % conversion) [1].
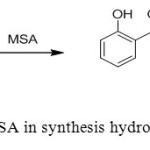 |
Figure8: Use of MSA in synthesis hydroxyacetophen one Click here to View figure |
9. Methane sulphonic acid used as catalyst for synthesis of 3-amino 2H-pyrazoles prepared by reaction of β-keto nitrile with hydrazine under solvent and solvent free conditions [9].
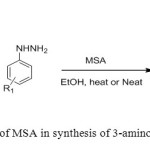 |
Figure9: Use of MSA in synthesis of 3-amino-2H-pyrazoles Click here to View figure |
10. Silica-supported methane sulphonic acid used as a solid acid catalyst for three component coupling reactions of indoles and sodiumnitrite to synthesize (E)-2, 3’-bi(3H-indol)-3-one oxime derivatives [10].
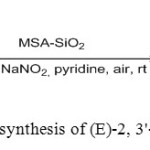 |
Figure10: Use of MSA for synthesis of (E)-2, 3′-bis(3H-indol)-3-one oxime Click here to View figure |
11. Methane sulphonic acid on silica was used as solid support catalyst for Pechmann reaction to synthesize Coumarin [11].
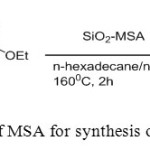 |
Figure11: Use of MSA for synthesis of Coumarin Click here to View figure |
12. R. Reddy Leleti et. al reported isomerisation of secondary and tertiary allylic alcohols undergo 1,3-isomerization using MSA under simple and efficient conditions to afford selectively the corresponding primary E-allylic alcohols in excellent yields [12].
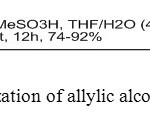 |
Figure12: Isomerization of allylic alcohols using MSA Click here to View figure |
13. M. Heravi et. al MSA used for catalyzes one-pot, three component reaction of an aromatic aldehyde, malonitrile and α or β-naphthol to yield 2-amino-4H-chromenes in very good yields[13].
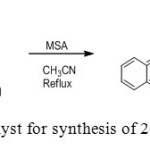 |
Figure13: MSA as a catalyst for synthesis of 2-amino-4H-chromenes Click here to View figure |
References
- Commarieua, A. ; Hoelderich, W.; Laffitte, J.; Dupont, M. J. Mol. Catalysis A: Chemical 2002, 182-183, 137-141.
- Naeimi, H.; Raeisi, A.; Moradian, M. Arabian Journal of Chemistry 2013, doi: http://dx.doi.org/10.1016/j.arabjc.2013.10.017
- Heravi, M.; Baghernejad, B.; Oskooie, H. Journal of the Chinese Chemical Society, 2008, 55, 659-662.
- Jhansi Rani, V.; Suresh, M.; Lavanya, P.; Vani, K.; Nagarjuna, B.; Venkata Rao, C. Der Pharma Chemica, 2010, 2, 224-230
- Kulkarni, P.; Wagh, P.; Zubaidha, P. Chemistry Journal 2012 2 106-110
- Sharghi, H.; Sarvari, M. Tetrahedron 2003, 59, 3627-3633
- Gora, M.; Kozik, B.; Jamrozy, K.; Luczynski, M.; Brzuzan, P.; Wozny, M. Green Chemistry 2009, 11, 863-867
- Borse, A.; Patil, M.; Patil, N.; Shinde, R. ISRN Organic Chemistry 2012, 2012, doi: 10.5402/2012/415645
- Suryakiran, N. ; Reddy, T.; Asha Latha, K.; Prabhakar, P.; Yadagiri, K. Journal of Molecular Catalysis A: Chemical 2006, 258, 371-375.
- Chang, Q.; Qu, H.; Qin, W.; Liu, L.; Chen, Z. Synthetic Communications 2013, 43, 2926-2935.
- Joshi, J.; Mishra, M.; Srinivasarao, M. Canadian Journal of Chemistry 2011, 89, 663-670.
- Reddy Leleti ,R.; Hu, B.; Prashad, M.; Repic, O. Tetrahedron Letters, 2007, 48, 8505-8507.
- Heravi, M.; Baghernejad, B.; Oskooie, H. Journal of the Chinese Chemical Society, 2008, 55, 659-662.

This work is licensed under a Creative Commons Attribution 4.0 International License.









