Synthesis of Functionalized Single Graphene Sheets by Thermal Exfoliation of Graphite Oxide
Abdul Jabar Mohmmed Saleh Al-Eyani3 and Nabil Abdullah Noman Alkadasi1,2,3
1Hubei key lab of Materials Chemistry & Service Failure , School of Chemistry & chemical engineering , Huazghog University of Science and Technology, Wuhan , Hubei , 430074 China . 2Department of chemistry ,Faculty of Education , , Al-baida'a ,University , Yemen , P.O.Box:39189 3Mechanical Engineering ,Faculty of Engineering ,Thamar university ,Yemen
DOI : http://dx.doi.org/10.13005/ojc/300405
Article Received on :
Article Accepted on :
Article Published : 09 Dec 2014
Synthesis is described to produce single sheets functionalized graphene through thermal exfoliation of graphite oxide .Synthesis yields a single sheet structure resulting from the reaction sites involved in oxidation and reduction process. Application of graphite used for unelectrical material and used pencil meanwhile main applications of graphene sheets are electrical materials.
KEYWORDS:Graphite; Graphene oxide and Graphene sheets; characterization and application
Download this article as:| Copy the following to cite this article: Al-Eyani A. J. M. S, Alkadasi N. A. N. Synthesis of Functionalized Single Graphene Sheets by Thermal Exfoliation of Graphite Oxide |
| Copy the following to cite this URL: Al-Eyani A. J. M. S, Alkadasi N. A. N. Synthesis of Functionalized Single Graphene Sheets by Thermal Exfoliation of Graphite Oxide. Available from: http://www.orientjchem.org/?p=5609 |
Introduction
Graphene is a single hexagonally flat layer of graphite, which has attracted great interest both for fundamental understanding of its unique structural and electronic properties and for important potential applications in nano-electronics and devices [1-5]. The unique properties of this two-dimensional (2D) material include the highest intrinsic carrier mobility at room temperature of all known materials and very high mechanical strength and thermal stability [6-9]. Graphene holds great promise for the development of new composite materials, emissive displays, ultrasensitive detectors and micromechanical resonators [10-12]. The combination of high mobility, thermal, chemical and mechanical stability with the high surface area offers many interesting applications in a wide range of fields including heterogeneous catalysis where metallic and bimetallic nano-particle catalysts can be efficiently dispersed on the graphene sheets [13-15]. In many cases, the remarkable properties of single graphene layers extend to bilayers and a few layers of graphene sheets. Several methods have been reported for the production of graphene sheets including micromechanical cleavage and thermal expansion of graphite.
Experimental
Materials
Physical parameters of Graphite 99.95% , Potassium Chlorite, Sulfuric acid H2SO4 and Nitric acid ( HNO3) ,99.9 % are reported in table 1, 2 ,3 and 4 respectively.
Table 1. General Characteristics of Graphite 99.95% .
| Trade Name | Graphite 99.95 % |
| Appearance | Black |
| Size | 325 mesh |
| Company | Aladdin Industrial Corporation Shanghai , China |
Table 2. General characteristics of Potassium Chlorate.
| Trade Name | KClO3 |
| Appearance | White |
| Molecular weight | 122.55 |
| Company | China |
Table 3. General Characteristics of Sulfuric Acid H2SO4.
| Molecular formula | Sulfuric Acid ( H2SO4) ,99.9 % |
| Appearance | liquid |
| Molecular weight | 98.08 |
| Concentration | 95 – 98 % |
| Company | Sinopharm chemical reagent Co ,Ltd ,China |
Table 4. General Characteristics of Nitric Acid ( HNO3) ,99.9 % .
| Molecular formula | Nitric Acid ( HNO3) ,99.9 % |
| Appearance | liquid |
| Molecular weight | 63.01 |
| Concentration | 65 – 68 % |
| Company | Sinopharm chemical reagent Co ,Ltd ,China |
Fabrication of Graphene Sheets
Commercial powdered natural graphite ( from Aladdin Industrial Corporation Shanghai , China ) was used as our starting material. The commercial graphite has a particle size of 325 mesh with a purity of 99.99% . Graphite was oxidized following modified Method of Staudenmaier method to form graphite oxide (GO). In this method, graphite (2.5g) was first mixed with sulfuric acid (43.75 mL) and nitric acid (22.5 mL) and stirred. When graphite was uniformly dispersed, potassium chlorate (27.5 g) was added slowly and stirred for over 96 h. After the completion of oxidation reaction, the mixture was added into 4 L deionized water and then filtered. The GO was repeatedly rinsed and redispersed in a 5% solution of HCl. It was then washed continually with deionized water until the pH of the filtrate was neutral [16-32]. Potassium stayed in the GO even after several times of washing .Therefore multiple washing cycles used in conjunction with bath ultrasonication in fresh ethanol were used for the removal of residual potassium . After this the GO was dried in a vacuum oven at 60 0C until used. Finally, the GO was treated with the nitrogen ( N2 ) and then it was heated to 1050 0C in the furnace for 30 s to form graphene sheets [16-31].
 |
Photo1: Graphite in water |
 |
Photo2: GO green before dry GO.dry in 60 0C water
|
 |
Photo3: Graphene Sheet in water
|
Treatment Silicon Wafer
silicon wafer were cut into ( 3mm x 3mm ) used Piranha solution is mixture consisting of sulfuric acid ( H2SO4 ) and hydrogen peroxide ( H2O2 ) is to cleaning silicon wafer . It is typically mixed in concentration ratios of around 3:1 H2SO4:H2O2 for one hour with temperature 60 0C . Then followed by triple rinsing in ethanol with ultrasonic cleaning for 30 min then with nitrogen ( N2 ) .There are two main applications for piranha in wafer fabrication: it is used to remove organic contaminants from surface of the wafer during cleaning . Silicon wafer were used for SEM [ 32].
Results and Discussion
The GO was reduced to graphene sheet by heating to 1150 0C. Plate 1 ,2( SEM ) and plate 7 ( TEM ) shows the top-view TEM images of the
graphite oxide plate ( SEM ) 3 , 4 and plate 8 ( TEM ) . TEM images of the original graphite plate ( SEM ) 5 ,6 and plate 9 ( TEM ) . A small flake of graphite particle is seen in .The size of the graphene sheet is about 9.35µm from SEM photo. The surface of graphene shows several large meanderingwrinkles. The thickness of graphene can be clear in high-magnification TEM image .
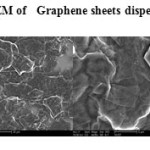 |
Plate1: SEM of Graphene sheets dispersed in water Click here to View plate |
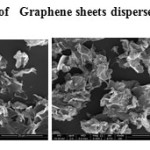 |
Plate2: SEM of Graphene sheets dispersed in ethanolClick here to View plate |
 |
Plate3: SEM of Graphene Oxide ( Dry ) dispersed in waterClick here to View plate |
 |
Plate4: SEM of Graphene Oxide ( Dry ) dispersed in ethanolClick here to View plate |
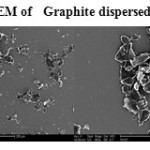 |
Plate5: SEM of Graphite dispersed in water Click here to View plate |
 |
Plate6: SEM of Graphite dispersed in ethanolClick here to View plate |
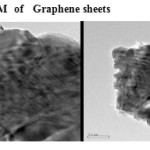 |
Plate7: TEM of Graphene sheets Click here to View plate |
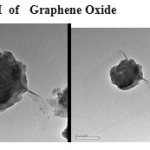 |
Plate8: TEM of Graphene Oxide Click here to View plate |
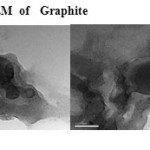 |
Plate9: TEM of GraphiteClick here to View plate |
Conclusions
single sheets functionalized graphene through thermal exfoliation of graphite oxide . Main application of graphene sheets in electrical material. The surface of graphene sheets shows several large meandering wrinkles. The thickness of graphene can be determined from the high-magnification TEM image.
Acknowledgements
I acknowledge the financial support from UNESCO/Government of China ( Great wall ) and Al-baida’a ,University , Yemen.
References
- K.S .Novoselov, A.K .Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V .Dubonos, et al. Electric field effect in atomically thin carbon films. Science 2004; 306: 666–9.
- Novoselov KS, Jiang D, Schedin F, Booth TJ, Khotkevich VV, Morozov SV, et al.Two-dimensional atomic crystals. Proc Natl Acad Sci USA 2005;102: 10451–3.
- Geim AK, Novoselov KS. The rise of graphene. Nat Mater 2007; 6:183–91.
- Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Twodimensional gas of massless Dirac fermions in grapheme. Nature 2005; 438: 197-202.
- Partoens B, Peeters FM. From graphene to graphite: electronic structure around the K point. Phys Rev B 2006; 74: 075404.
- Ghorbani, H.R., Orient J Chem., 2014, 30(2), 803-806.
- Graf D, Molitor F, Ensslin K, Stampfer C, Jungen A, Hierold C, et al. Spatiallyresolved Raman spectroscopy of single- and few-layer graphene. Nano Lett 2007;7:238–42.
- Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, et al. Graphene-based composite materials. Nature 2006; 442: 282–6.
- McAllister MJ, Li JL, Adamson DH, Schniepp HC, Abdala AA, Liu J, et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite.Chem Mater 2007;19: 4396–404.
- Stankovich S, Piner RD, Nguyen ST, Ruoff RS. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006; 44: 3342–7.
- Stankovich S, Piner RD, Chen X, Wu N, Nguyen ST, Ruoff RS. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J Mater Chem 2006; 16: 155–8.
- Li X, Zhang G, Bai X, Sun X, Wang X, Wang E, et al. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat Nanotechnol 2008; 3: 538–42.
- Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun ZY, De S, et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 2008; 3: 563–9.
- Lotya M, Hernandez Y, King PJ, Smith RJ, Nicolosi V, Karlsson LS, et al. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J Am Chem Soc 2009; 131: 3611–20.
- Pu NW, Wang CA, Sung Y, Liu YM, Ger MD. Production of few-layer graphene by supercritical CO2 exfoliation of graphite. Mater Lett 2009;63:1987–9.
- Liu N, Luo F, Wu H, Liu Y, Zhang C, Chen J. One – step ionic – liquid – assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater 2008;18:1518–25.
- Li D, Mu¨ ller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersion of graphene nanosheets. Nat Nanotechnol 2008; 3:101–5.
- Liu Z, Robinson JT, Sun X, Dai H. PEGylated nano-graphene oxide for delivery of water insoluble cancer drugs. J Am Chem Soc 2008;130:108767.
- Wu H, Wang J, Kang X, Wang C, Wang D, Liu J, et al. Glucose biosensor based on immobilization of glucose oxidase in platinum nanoparticles/graphene/chitosan nanocomposite film. Talanta 2009; 80(1): 403-6.
- Stoller MD, Park S, Zhu Y, An J, Ruoff RS. Graphene-based ultracapacitors. Nano Lett 2008; 8: 3498–502.
- Hsiao MH, Liao SH, Yen MY, Teng CC, Lee SH, Pu NW, et al. Preparation and properties of a graphene reinforced nanocomposite conducting plate. J Mater Chem 2010; 20:8496–505.
- Liu Y, Ren L, He Y, Cheng HP. Titanium-decorated graphene for high-capacity hydrogen storage studied by density functional simulations. J Phys Condens Matter 2010; 22: 445301.
- Schniepp HC, Li JL, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH, et al.Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 2006;110: 8535–9.
- Viculis LM, Mack JJ, Mayer OM, Hahn HT, Kaner RB. Intercalation and exfoliation routes to graphite nanoplatelets. J Mater Chem 2005; 15: 974–8.
- Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, et al.Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 2009; 323: 610–3.
- Rao CNR, Biswas K, Subrahmanyam KS, Govindaraj A. Graphene, the new nanocarbon. J Mater Chem 2009;19:2457–69.
- Saito R, Fujita M, Dresselhaus G, Dresselhaus MS. Electronic structure of chiral graphene tubules. Appl Phys Lett 1992; 60: 2204–6.
- Liang Y, Wu D, Feng X, Mu¨ llen K. Dispersion of graphene sheets in organic solvent supported by ionic interactions. Adv Mater 2009;21:1679–83.
- Li D, Muller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 2008; 3: 101–5.
- Paredes JI, Villar-Rodil S, Mart1´o´ nez-Alonso A, Tasco´n JMD. Graphene oxide dispersions in organic solvents. Langmuir 2008; 24:10560–4.
- Li JT, Li M, Li JH, Sun HW. Removal of disperse blue 2BLN from aqueous solution by combination of ultrasound and exfoliated graphite. Ultrason Sonochem 2007;14:62–6.
- Shen J, Hu Y, Li C, Qin C, Ye M. Synthesis of amphiphilic graphene nanoplatelets. Small 2009; 5: 82–5.
- Yizhi W, Cheng Z, Ye Y, Ziwen W, Weijia S, Huijie W and Xiaoliang X. Fabrication of wafer – Zize Monolayer close –packed colloidal crystals via slope self –assembly and thermal treatment . Langmuir 2013; 29: 14017-14023.

This work is licensed under a Creative Commons Attribution 4.0 International License.









