Synthesis, Characterization and Thermal Properties of Fe2TiO5/Cellulose and Cellulose Acetate Nanocomposite
S. Khanahmadzadeh and Gh. Hosseiny
Department of Chemistry, Mahabad Branch , Islamic Azad University , Mahabad , Iran
DOI : http://dx.doi.org/10.13005/ojc/300457
Article Received on :
Article Accepted on :
Article Published : 16 Dec 2014
Cellulose and cellulose acetate/Fe2TiO5 nanocomposites have been synthesized successfully. Nanocomposites indicate a ferromagnetic paramagnetic behavior, as evidenced by using vibrating sample magnetometer (VSM) at room temperature. Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM) and simultaneous thermal analysis (STA) respectively, to characterize,diagnosis, morphology and particle size, and to measure the thermal properties of samples. STA investigations reveal that the thermal stability of the cellulose and cellulose acetate is significantly enhanced with the addition of nano fillers. The results indicated that Fe2TiO5nanopowders were distributed in cellulose and cellulose acetate matrix with particle size between 25 and 75 nm.
KEYWORDS:cellulose acetate; nanocomposite; STA; VSM
Download this article as:| Copy the following to cite this article: Khanahmadzadeh S, Hosseiny GH. Synthesis, Characterization and Thermal Properties of Fe2TiO5/Cellulose and Cellulose Acetate Nanocomposite. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Khanahmadzadeh S, Hosseiny GH. Synthesis, Characterization and Thermal Properties of Fe2TiO5/Cellulose and Cellulose Acetate Nanocomposite. Available from: http://www.orientjchem.org/?p=5902 |
Introduction
At present, cellulose is the most abundant polymer available worldwide with an estimated annual natural production of1.5 × 1012 tons and considered as an almost inexhaustible source of raw based materials because of the biodegradability and renewable aspects of these materials [3]. Acetylation of cellulose with acetyl chloride or acetic anhydride has been known for a long time. Cellulose acetate is one of the most commercially important cellulose derivatives. It is widely used in textiles because of its low cost, toughness, gloss, high transparency, natural feel, and other favorable aesthetic properties. Cellulose acetate fibers in cigarette filters are designed to absorb vapors and accumulate particulate smoke components. Cellulose acetate is also used as a carrier for photographic negatives, motion picture film (celluloid), microfilm, microfiche, and audio tape [3-5]. In addition, the acetylation of cellulose is also widely used for the protection of hydroxy groups and the purification and structural elucidation of natural products. Various methods have been developed for producing cellulose acetates, in which acetic anhydride and acetyl chloride are commonly used as acetylating reagents. Industrially, cellulose acetates are often produced by reaction of cellulose with an excess of acetic anhydride in the presence of sulfuric acid or perchloric acid as the catalysts [6]. Titanium-substituted iron oxides are widespread in nature and represent an important mineral resource for the commercial extraction of both iron and titanium [7]. Mixed metaloxides have been widely investigated since they have interesting catalytic properties. Chemical properties of the active sites can be significantly modified by mixing oxides, in the form of solid solutions, or by supporting the oxide catalyst on another oxide .There has been growing interest in iron titanium oxide as a good candidates for new optical fibers, and as a sensor material for the detection of NO2 [8] and gas sensing applications.The polycrystalline ferrites have been studied for several years due to their wide use as magnetic materials for telecommunications audio and video power transformers and many other applications. The crystallographic, electrical and magnetic properties depend strongly on the stoichiometry, preparation conditions and particle size. In this study, cellulose and cellulose acetate /Fe2TiO5 nanocomposites with different cellulose and cellulose acetate: Fe2TiO5 ratios (10% and 20%) were prepared and their Synthesis, characterization and thermal properties was investigated. The composites made from mineral and polymeric compounds,cover their disadvantage. And produce less flammable, more mechanical and biodegradable products [9]. The properties of ceramic and polymeric composites are function of properties of reagents. In the other hand they depend on composition percent,the size of ingredients and the manner of components scattering in the composite [10]. The most studies were carried out on derivatived polymers from oil resources such as poly phenylene sulfide, cyclic olefin, copolymer, epoxy and acryl derivatives and very limited researches have been accomplished on properties of composites made from ceramic compounds and biodegradable polymers. Finally it is worth mentioning that all of the composite materials could be magnetized. Thus, they could possibly be used in advanced separation processes induced by external magnetic field (separation due to pore structure and due to magnetism), for controlled drug delivery or in more common applications of polymer–iron oxide composites such as sound recording media (magnetic tapes).
Experimental
Cellulose and cellulose acetate /Fe2TiO5 nanocomposites were prepared along a synthetic procedure as summarized in Fig.1. Materials used in this work were Iron acetyl acetonate, tetrabutyl titanate, stearic acid; cellulose and cellulose acetate were all of Merck. Fe2TiO5 nanoparticles were prepared by a modified wet-chemistry synthesis method which is described in the literature [11]. In this way, a fixed amount of Iron acetyl acetonate was added to the melted stearic acid and dissolved. Then, stoichiometric tetrabutyltitanate was added to the solution, stirred to form sol, naturally cooling down to room temperature, and being dried to obtain dried gel. Finally, the gel was calcined at 900°C in air to obtain nanopowders of Fe2TiO5.
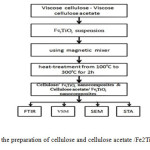 |
Fig1: Flow chart for the preparation of cellulose and cellulose acetate /Fe2TiO5 nanocomposites.
|
The quantities of ceramic nano particles in the composite were 10 and 20 volume percent respectively .In order to prepare the desired composites,at first ,the cellulose and cellulose acetate were weighed in the base of their volume percent of specific weight .calculations,then they were placed in to a beaker .In order to uniform distribution of ceramic nano particles in the blank ,the polymer was solvated. In this stage the pure acetone (99 % purity) was used as suitable solvent for cellulose and cellulose acetate .After complete dissolution of cellulose polymer in the acetone ,ceramic nano particles were added to solution in the base of their calculated volume percent.The solution was stirred with electrical stirrer to produce an uniform mixture.In the stage of solvent vaporing, in order to homo scattering of ceramic nano particles in cellulose blank ,the ultra-sonicapparatuse was applicated. After solvent vaporing, the prepared combined compounds, was grinded to obtain the monotone powder. The formation of process and structural characterization of cellulose and cellulose acetate / Fe2TiO5 phases have been investigated by STA, FTIR, VSM and SEM. Simultaneous Thermal Analysis experiments were performed by STA(model of PL-STA-1640) in air to investigate the calcinations temperature and possible phase transformation from 25°C to 500 °C with a heating rate of 5 °C/min. The FTIR spectrum was recorded with a model perkin Elmer spectrum RX1of Fourier taransform infrared spectrometry by using KBr pellet. The SEM pictures were recorded with KYKY Model EM 3200 instrument at the accelerating voltage of 25Kv. Magnetisation measurement is carried out using vibrating sample magnetometer (VSM; BHV-55, Riken, Japan) at room temperature.
Results and Discussion
Ft-Ir Analysis
Fig. 2 and Fig.3 show the FT-IR spectras of the cellulose / Fe2TiO5 nanocomposite of 10% and 20% and cellulose acetate /Fe2TiO5 nanocomposite of 10% and 20% respectively.A band at 3429 cm_1 in Figs. 1 and 2 is attributed to the O–H stretching vibration of hydroxyl group of cellulose and cellulose acetate. The peak due to the aliphatic C–H stretching vibrations appeared around 2931 cm_1. The absorption peak observed around 1722 cm_1 may be due to the carbonyl (C=O)stretching vibration of the α-keto carbonyl present in the cellulose and cellulose acetate thus confirming the reaction between the cellulose and cellulose acetate and the Fe2TiO5. Furthermore, the bands around 1429 cm_1, are associated with C-H in planedeformation of C-H groups of the cellulose and cellulose acetate. While the bands around 1033 cm_1 involve the C–O stretching vibrations of aliphatic primary and secondary alcohols in cellulose and cellulose acetate. In this spectrums, the absorption peaks under the 800 cm−1 is assigned to Fe-O band of Fe2TiO5 nanoparticles.
 |
Fig2: FT-IR spectra of (a) cellulose ,(b) cellulose /Fe2TiO5 nanocomposite with a nanoparticle loading of 10 wt%,(c) cellulose /Fe2TiO5 nanocomposite with a nanoparticle loading of 20 wt%. Click here to View figure |
 |
Fig3: FT-IR spectra of (a) cellulose acetate ,(b) cellulose acetate /Fe2TiO5 nanocomposite with a nanoparticle loading of 10 wt%,(c) cellulose acetate /Fe2TiO5 nanocomposite with a nanoparticle loading of 20 wt% Click here to View figure |
Morphology of Samples
The morphology of pure Fe2TiO5 and nanocomposites were evaluated by SEM to observe the distribution of nanoparticles within the extruded materials. Fig. 4. shows micrographs of a cryofractured surface of the nanocomposites. Moderate nanoparticle dispersion is seen in these micrographs. However, in spite of using acetone for preventingthe coalescence of nanoparticles, partial coalescence was unavoidable especially at higher nanoparticle content. As seen in Fig. 4c and e, many particles were larger than 40–60 nm (original size). These particles had undergone coalescence and a coarse coalescence was seen for cellulose and cellulose acetate /Fe2TiO5 nanocomposites of 20 wt%. A good dispersion of nanofillers within the cellulose and cellulose acetate matrix could change the dynamic mechanical properties of the nanocomposites even at lower nanofiller loadings owing to enhanced filler-matrix interaction. This is due to the intrinsic properties of the inorganic nanofillers.The surface morphology of the obtained nanocomposites was investigated by scanning electron microscopy(SEM). Particles have agglomerated graining structure. Scanning electron microscopy (SEM) characterizations reveal the uniform coating of polymer on the filler surface and a good dispersion of the nanofillers within the polymer matrix.The particle size was estimated between 25-75 nm.
 |
Fig4: SEM images of (a) Fe2TiO5 nanoparticles (b) Cellulose/Fe2TiO5 nanocomposite with Fe2TiO5 content of (10) wt% and (c) (20) wt% (d) Cellulose acetate/Fe2TiO5 nanocomposite with Fe2TiO5 content of (10) wt% and (e) (20) wt%. Click here to View figure |
Thermal Analysis
Fig. 5. shows the STA curves of cellulose and cellulose acetate /Fe2TiO5 nanocomposites. The thermogram of pure cellulose and cellulose acetate shows that the initial mass loss is due to the loss of water molecules, the very next mass loss may be attributed to the loss of oligomer and the subsequent rapid mass loss is occurred due to the degradation of the polymer chain. But in cellulose and cellulose acetate/Fe2TiO5 nanocomposites mass loss occur slowly up to 500 ◦C due to removal of dopantmolecules from the polymer structure. Rapid mass loss occurs at around 500 ◦C due to rapid degradation of polymer chain. This thermogravimetric analysis indicates better thermal stability of the cellulose and cellulose acetate /Fe2TiO5 nanocomposites than that of pure cellulose and cellulose acetate. The better thermal stability of cellulose and cellulose acetate/Fe2TiO5 composite can be explained by the dominancy of the benzenoid structure. In both of two Figures, the first weight losses in the temperature range from 50°C to 100°C, is attributed to the elimination of moisture and adsorbed water in the samples. The major weight loss of all the samples From250°C to 500 °C is due to a thermal decomposition of the Organic substances, that two peaks exist in 300°C and 450°C, confirm it. By comparison between Fig.a. and Fig.b. , results indicated that STA curves of two samples are not very different. The weight loss curve of 20% sample has the lower (decline), since in this sample, the amount of polyaniline is lower than the 10% sample. (This result indicates that the thermal stability of polyaniline/Fe2TiO5 nanocomposites is improved with the addition of Fe2TiO5 nanoparticles.).
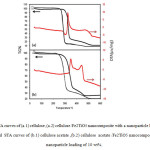 |
Fig5: STA curves of (a.1) cellulose ,(a.2) cellulose /Fe2TiO5 nanocomposite with a nanoparticle loading of 10 wt% and STA curves of (b.1) cellulose acetate ,(b.2) cellulose acetate /Fe2TiO5 nanocomposite with a nanoparticle loading of 10 wt%. Click here to View figure |
Magnetic Properties
Fig. 6 shows the field dependent magnetization curves of Fe2TiO5, cellulose and cellulose acetate / Fe2TiO5 nanocomposite of 20wt%. Iron titanates are reported as ferrimagnetic materials [12], but specifically an antiferromagnetic ordering with weak ferromagnetism has been predicted for Fe2TiO5 [13]. Under applied magnetics field Fe2TiO5, and cellulose and cellulose acetate / Fe2TiO5 nanocomposites show the positive magnetizations. It indicates the ferromagnetic behavior for Fe2TiO5, cellulose and cellulose acetate / Fe2TiO5 nanocomposites of 20wt% and their magnetization values are 0.7, 0.03 and 0.13 emu/g, respectively. With increase of Fe2TiO5 content in cellulose and cellulose acetate, magnetization increases. The variation of magnetization value is due to different amounts of Fe2TiO5 in cellulose and cellulose acetate.
 |
Fig6: VSM curves of (a) Fe2TiO5 (b) Cellulose /Fe2TiO5 (20 wt%) and (c) Cellulose acetate/Fe2TiO5 (20 wt%). Click here to View figure |
Conclusions
In this research ,two kinds of composite were synthesized from two biodegradable polymers.These polymers are derivated from reproducible resources:cellulose and cellulose acetate with Fe2TiO5 nanoparticles as ceramic nanoparticles.Results ,show that ,the process of nanoparticles produce and composits,with combination of various percents is possible and leads to prepare of compact composits with equal distribution of ceramic nanoparticles .In IR spectroscopy comment of nano composite the presence of all elements are specified using the pick positions and references. The results of scanning electron microscopy (SEM) ,showed the nano composits in the scale of micro. Thermo analysis showed that no pick it means that all kinds of organic compounds were decomposed was observated at high ten perture up to 509°c. This study also showed, the synthesised cellulose / Fe2TiO5 nanocomposite has indicated a ferrimagnetic paramagnetic behaviour, as evidenced by using VSM at room temperature. Simultaneous thermal Analysis (STA) reveals an enhanced thermal stability of the nanocomposites with the addition of nanoparticles as compared to that of pure cellulose and cellulose acetate.
References
- Klemm, D. Heublein, B. Fink, H.P. Bohn, A. Chem. Int. Ed. 44,3358–3393.
- Cao, Y. Wu, J. Zhang, J. Li, H. Q. Zhang, Y. He, J. S. Chem. Eng.J.2009, 147, 13-21.
- Biswas, A. Shogren, R. L. Willett, J. L.2005, 6, 1843–1845.
- Edgar, K. J. Buchanan, C. M. Debenham, J. S. Rundquist, P.A. Seiler, B. D. Shelton, M. C. Tindall, D.Prog. Polym. Sci. 2001, 26, 1605–1688.
- Biswas, A. Selling, G. Appell, M. Woods, K. K. Willett, J. L. Buchanan, C. M., Polym. 2007, 68, 555–560.
- Hummel, A., Macromol. Symp. 2003, 208, 61–79.
- Taylor, RW. Am. mineral.1964, 49, 1016-1030.
- Bein, T. Brown, K. Fye, G. C. and Brinker, C. J. J. Am. Cer. Soc. 1989, 111, 7640-7641.
- Shokrieh , M. and Sonbolestan, S. E.Iran. J. Polym. Sci. Technol.2007, 20, 187-195.
- Mortezaei, M. Navid Famili, M.H. and Kokabi, M.J. Polym. Sci. Technol. 2009, 21, 523-53.
- Enhessari, M. Kargarrazi, M. Etemad, L. Parviz, A. Sakhaei, M.Journal of Experimental Nanoscience. 2011,1-24.
- Ishikawa, Y.J. Phys. Soc. Japan.1957, 12(10), 1083-1098.
- Hanel, D. Sevek, F.Mater.Res. Bull. 1984, 1935-39.

This work is licensed under a Creative Commons Attribution 4.0 International License.









