Synthesis, Antimicrobial and Antitubercular Activities of Some Novel Carboxamide Derivatives of 2-Quinolones
Abhishek Kumar*, Jennifer Fernandes and Pankaj Kumar
Department of Pharmaceutical Chemistry, NGSM Institute of Pharmaceutical Sciences , Nitte University, Paneer, Deralakatte-575018, Mangalore, Karnataka.
DOI : http://dx.doi.org/10.13005/ojc/300462
Article Received on :
Article Accepted on :
Article Published : 13 Nov 2014
A series of novel substituted N-(3-acetyl-2-oxoquinolin-1(2H)-yl)benzamide (AJQC1-AJQC12) have been synthesized upon refluxing 3-acetyl-1-amino-quinolin-2-one and substituted benzoic acid in the presence of dry redistilled pyridine and silicon tetra chloride as coupling agent. 3-acetyl-1-amino-quinolin-2-one (AJQ1-AJQ12) were synthesized from substituted 3-acetyl coumarin upon refluxing with hydrazine hydrate and ethanol. The structures of the final carboxamide derivatives were confirmed by IR, 1H NMR and mass spectra. The synthesized compounds were screened for their antimicrobial activity by tube dilution method and anti tubercular activity by microplate Alamar blue assay. Most of the compounds have exhibited promising antibacterial, anti fungal and anti tubercular activities.
KEYWORDS:2-Quinolones; carboxamide; antimicrobial activity; minimum inhibitory concentration; antitubercular activity
Download this article as:| Copy the following to cite this article: Kumar A, Fernandes J, Kumar P. Synthesis, Antimicrobial and Antitubercular Activities of Some Novel Carboxamide Derivatives of 2-Quinolones. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Kumar A, Fernandes J, Kumar P. Synthesis, Antimicrobial and Antitubercular Activities of Some Novel Carboxamide Derivatives of 2-Quinolones. Orient J Chem 2014;30(4). Available from: http://www.orientjchem.org/?p=5318 |
INTRODUCTION
2-Quinolones (carbostyrils or 1-aza coumarins) are isosteric with coumarins and isomeric to 4-quinolones could become the probable potential candidate for antibacterial activity (1). 2-Quinolone derivatives were found to be associated with various biological activities such as antitumor (2), anti-inflammatory (3), antiplatelet, antitubercular (4), antioxidant (5) and antidepressant activity. Pyrazinamide is a nicotinamide analogue that has been used as a first-line drug to treat tuberculosis (6). Various compounds possessing the –NHCO– group were found to inhibit photosynthetic electron transport (7). Amides are usually stable, neutral and have both hydrogen-bond acceptor/donator properties which are very important for the synthesis of versatile heteroaromatic molecules. The amide functionality is the common backbone of numerous organic molecules and natural products that bear diverse chemical and pharmacological features (8). A series of quinoline carboxamide derivatives have also been evaluated for positive inotropic activity (9), as potential radio ligands in the neurodegeneration systems (10) and antiviral against acyclovir resistant herpes simplex virus (11). By considering the above potent pharmacological properties of these quinoline ring containing compounds, it was contemplated to synthesize some novel 2-quinolone carboxamide derivatives. The final synthesized compounds were evaluated for their in vitro antimicrobial and anti tubercular activities and compared with standard drugs.
MATERIAL AND METHODS
All the chemicals were of analytical grade: substituted salicylaldehyde, ethylacetoacetate, absolute ethanol, piperidine, hydrazine hydrate, substituted benzoic acid, silicon tetrachloride and dry redistilled pyridine. Melting points were determined by open capillary method and are uncorrected. The purity of the compounds was monitored by thin layer chromatography (TLC) using silica gel G plates. The spots were visualized under UV light and by the exposure to iodine vapors. The homogeneity of the compounds were checked on silica gel-G coated plate by using Chloroform: Methanol (7:3) as solvent. All IR spectra were recorded in Alpha Bruker using ATR method. 1H NMR spectra were recorded on Bruker spectrophotometer (400 MHz) in DMSO-d 6 solvent using tetra methyl silane (TMS) as an internal standard. Mass spectra was recorded by LCMS method.
General Procedure
Synthesis of substituted 3-acetyl-1-amino-quinolin-2-one (AJQ1-AJQ12) (12)
Substituted 3-acetyl coumarin (0.01 mol) with excess hydrazine hydrate 99% (0.1 mol) in 25 ml ethanol was refluxed for 12 hours. It was then cooled and poured into crushed ice with stirring. The solid product formed was filtered and recrystallised from ethanol.
Synthesis of Substituted N-(3-acetyl-2-oxoquinolin-1(2H)-yl)benzamide (AJQC1- AJQC12) (13).
A mixture of substituted 3-acetyl-1-amino-quinolin-2-one (0.01 mol) and substituted benzoic acid (0.01 mol) was refluxed for 24 hours with stirring in 10 ml of dry redistilled pyridine and silicon tetrachloride as coupling reagent. After the completion of the reaction, the reaction mixture was poured into crushed ice. The precipitated solid was filtered, washed with cold water and recrystallised from ethanol.
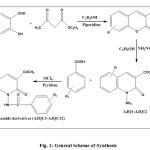 |
Fig1: General Scheme of Synthesis |
R: H, 6-NO2, 6-Cl
R1: H, 2-Cl, 4-Cl, 2-OH, 4-N(CH3)2, 2 NH
Spectral data
3-acetyl-1-aminoquinolin-2(1H)-one (AJQ1)
IR (cm-1)
1506(Ar C=C str), 829 (Ar C-H bend), 2950(C-H aliphatic str), 1701 (C=O str), 3362, 3398 (N-H str).
1H NMR
(400 MHz, DMSO-d6): δ 7.25-8.27 (m, 5H, Ar-H), 3.73(s, 2H, NH2), 2.59 (s, 3H, COCH3).
Mass (m/z)
202 (M+).
N-(3-acetyl-2-oxoquinolin-1(2H)-yl)benzamide (AJQC1)
IR (cm-1)
1511(Ar C=C str), 832 (Ar C-H bending), 3030 (Ar C-H str), 1696 (C=O str), 3160 (NH str 2˚amide).
1H NMR
(400 MHz, DMSO-d6) : δ7.21-7.92 (m, 10H, Ar-H), 10.13 (s, 1H, -NHCO-), 2.59 (s, 3H, COCH3).
Mass (m/z)
306 (M+).
N-(3-acetyl-6-nitro-2-oxoquinolin-1(2H)-yl)-2-chlorobenzamide (AJQC7)
IR (cm-1)
1508(Ar C=C str), 836 (Ar C-H bending), 3028 (Ar C-H str), 1692 (C=O str), 3158 (NH str 2˚amide), 1356 (Ar-NO2), 730 (C-Cl).
1H NMR
(400 MHz, DMSO-d6) : δ7.12-7.87 (m, 8H, Ar-H), 10.24 (s, 1H, -NHCO-), 2.54 (s, 3H, COCH3).
Mass (m/z)
386 (M+1).
N-(3-acetyl-6-chloro-2-oxoquinolin-1(2H)-yl)-2-aminobenzamide (AJQC12)
IR (cm-1)
1502(Ar C=C str), 830 (Ar C-H bending), 3032 (Ar C-H str), 1690 (C=O str), 3152 (NH str 2˚amide), 3360 (N-H str), 732 (C-Cl).
1H NMR
(400 MHz, DMSO-d6) : δ7.12-7.85 (m, 8H, Ar-H), 10.19 (s, 1H, -NHCO-), 3.72(s, 2H, NH2), 2.52 (s, 3H, COCH3).
Mass (m/z)
356 (M+1).
Antimicrobial Activity
All the synthesized compounds were evaluated for their minimum inhibitory concentration by tube dilution method (14). The synthesized test compounds were tested at different concentrations and ciprofloxacin and fluconazole was used as standard. Serial dilutions of the test compound was made in a liquid medium which was inoculated with a standardized number of organisms and incubated for 24 hrs. The lowest concentration of test compound preventing appearance of turbidity is considered to be the minimal inhibitory concentration (MIC). After preparation of different concentrations of the antimicrobial agent in brain heart infusion broth (by using the broth dilution method), we inoculate them with the tested organism. Then after incubation we can determine the MIC by choosing the lowest concentration in which no growth occurs.
Antitubercular Activity
The antitubercular activity of test compounds were assessed against Mycobacterium tuberculosis using microplate Alamar blue assay (15). This methodology is non toxic, uses a thermally stable reagent and shows good correlation with proportional and BACTEC radiometric method. 200 µl of sterile deionized water was added to all outer perimeter wells of sterile 96 well plate to minimised evaporation of medium in the test wells during incubation. The 96 well plate received 100 µl of the Middle brook 7 H9 broth and serial dilution of compounds were made directly on plate. The final drug concentrations of the tested compounds were 0.01 to 20.0 µl/ml. The plates were covered and sealed with parafilm and incubated at 37οC for 5 days. After this, 25 µl of freshly prepared 1:1 mixture of Alamar blue reagent and 10% tween 80 was added to the plate and incubated for 24 hours. A blue color in the well was interpreted as no bacterial growth and pink color was interpreted as growth. The minimum inhibitory concentration was defined as lowest drug concentration which prevented the color change from blue to pink.
RESULTS
Table 1: Physicochemical data of the compounds AJQC1-AJQC12
|
Comp. code |
R |
R1 |
Mol. formula |
Mol. wt |
M.P oC |
Rf Value |
% Yield |
|
AJQC1 |
H |
H |
C18H14N2O3 |
306 |
172-174 |
0.58 |
78 |
|
AJQC2 |
H |
2-Cl |
C18H13ClN2O3 |
340 |
180-182 |
0.70 |
72 |
|
AJQC3 |
H |
4-Cl |
C18H13ClN2O3 |
340 |
188-190 |
0.62 |
75 |
|
AJQC4 |
H |
2-OH |
C18H14N2O4 |
322 |
196-198 |
0.56 |
70 |
|
AJQC5 |
H |
4-N(CH3)2 |
C20H19N3O3 |
349 |
184-186 |
0.68 |
73 |
|
AJQC6 |
H |
2-NH2 |
C18H15N3O3 |
321 |
206-208 |
0.60 |
76 |
|
AJQC7 |
6-NO2 |
2-Cl |
C18H12ClN3O5 |
385 |
190-192 |
0.74 |
62 |
|
AJQC8 |
6-NO2 |
2-NH2 |
C18H14N4O5 |
366 |
212-214 |
0.64 |
58 |
|
AJQC9 |
6-Cl |
4-Cl |
C18H12Cl2N2O3 |
374 |
202-204 |
0.76 |
65 |
|
AJQC10 |
6-Cl |
2-OH |
C18H13ClN2O4 |
356 |
220-222 |
0.70 |
68 |
|
AJQC11 |
6-Cl |
4-N(CH3)2 |
C20H18ClN3O3 |
383 |
208-210 |
0.68 |
60 |
|
AJQC12 |
6-Cl |
2-NH2 |
C18H14ClN3O3 |
355 |
226-228 |
0.72 |
66 |
Table 2: Minimum inhibitory concentration of the compounds (AJQC1-AJQC12) by tube dilution method
|
Comp Code |
Minimum inhibitory concentration (µg) |
|||||
|
B. subtilis |
S. aureus |
E.coli |
P.aeruginosa |
C.albicans |
A.niger |
|
|
AJQC1 |
3.2 |
12.5 |
1.6 |
25 |
12.5 |
100 |
|
AJQC2 |
1.6 |
1.6 |
R |
R |
25 |
R |
|
AJQC3 |
100 |
R |
1.6 |
1.6 |
R |
R |
|
AJQC4 |
50 |
50 |
25 |
50 |
12.5 |
3.2 |
|
AJQC5 |
25 |
R |
6.25 |
0.8 |
50 |
6.25 |
|
AJQC6 |
3.2 |
1.6 |
6.25 |
50 |
1.6 |
100 |
|
AJQC7 |
3.2 |
6.25 |
25 |
12.5 |
R |
100 |
|
AJQC8 |
3.2 |
50 |
12.5 |
0.8 |
50 |
3.2 |
|
AJQC9 |
100 |
50 |
50 |
100 |
3.2 |
6.25 |
|
AJQC10 |
12.5 |
1.6 |
100 |
100 |
6.25 |
6.25 |
|
AJQC11 |
25 |
50 |
6.25 |
1.6 |
100 |
100 |
|
AJQC12 |
3.2 |
1.6 |
25 |
12.5 |
6.25 |
3.2 |
|
Ciprofloxacin |
1 |
2 |
2 |
1 |
|
|
|
Fluconazole |
|
|
|
|
16.6 |
8.3 |
Table 3: Antitubercular activity of compounds (AJQC1-AJQC12) by Microplate Alamar blue assay
|
Comp. Code |
R |
R1 |
MIC in µg |
|
AJQC1 |
H |
H |
50 |
|
AJQC2 |
H |
2-Cl |
25 |
|
AJQC3 |
H |
4-Cl |
25 |
|
AJQC4 |
H |
2-OH |
6.25 |
|
AJQC5 |
H |
4-N(CH3)2 |
3.125 |
|
AJQC6 |
H |
2-NH2 |
6.25 |
|
AJQC7 |
6-NO2 |
2-Cl |
50 |
|
AJQC8 |
6-NO2 |
2-NH2 |
6.25 |
|
AJQC9 |
6-Cl |
4-Cl |
25 |
|
AJQC10 |
6-Cl |
2-OH |
3.125 |
|
AJQC11 |
6-Cl |
4-N(CH3)2 |
12.5 |
|
AJQC12 |
6-Cl |
2-NH2 |
6.25 |
|
Standard |
INH |
0.2 |
|
|
Streptomycin |
6.25 |
||
DISCUSSION
Antimicrobial Activity
The final synthesized compounds (AJQC1-AJQC12) were screened for their minimum inhibitory concentration by tube dilution method. Compounds AJQC2, AJQC6, AJQC7 and AJQC12 showed significant antibacterial activity against gram +ve bacteria and compounds AJQC3, AJQC5 and AJQC11 showed significant antibacterial activity against gram -ve bacteria. Compounds AJQC1, AJQC4, AJQC6, AJQC9, AJQC10 and AJQC12 showed significant antifungal activity against C.albicans and compounds AJQC4, AJQC5, AJQC8, AJQC9, AJQC10 and AJQC12 showed significant antifungal activity against A.niger. The results of the minimum inhibitory concentration are summarized in Table 2.
Anti tubercular Activity
The test compounds (AJQC1- AJQC12) were evaluated for their anti tubercular activity against M.tuberculosis using Microplate Alamar Blue assay. A blue colour in the well was interpreted as no bacterial growth and pink colour was scored as growth. Most of the test compounds AJQC4, AJQC5, AJQC6, AJQC8, AJQC10, AJQC11 and AJPY12 showed promising antitubercular activity compared to the standard drug streptomycin and isoniazid. The presence of 2-quinolone moiety with electron donating groups like methyl, hydroxy, amino and dimethylamino has accounted for their antitubercular activity. The results of the antitubercular activity are summarized in Table 2.
CONCLUSION
This study reports the successful synthesis of carboxamide derivatives of 2-Quinolone moiety with moderate yields and most of the synthesized compounds showed potent antimicrobial and antitubercular activity.
ACKNOWLEDGEMENT
The authors are thankful to Nitte University for providing the necessary facilities to carry out this research. The authors are grateful to Sequent Research Ltd, Mangalore and Central Instrumentation Facility, MIT Manipal for providing spectral data.
REFERENCES
- Milecki, J.; Baker, S.P.; Standifer, K.M.; Ishizu, T.; Chida, Y.; Kusiak, J.W. J. Med. Chem. 1987, 30, 1563-1566
- Joseph, B.; Darro, F.; Behard, A.; Lesur, B.; Frydman, A.; Kiss, R. J. Med. Chem. 2002, 45, 2534-2555
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A.; Tkach, A.A.; Gorokhova, O.V. Chem. Heterocycl. Compd. 2012, 46(8), 974-986
- Musica, G.C.; Bollini, M.; Bruno, A.M.; Asis, S.E. J. Chil. Chem. Soc. 2006, 51(2), 859-863
- Jayashree, B.S.; Thomas, S.; Nayak, Y. Med. Chem. Res. 2010, 19(2), 193-209
- Snider, D.E.; Castro, K.G. New. Eng. J. Med. 1998, 338, 1689-1690.
- Good, N.E. Plant. Physiol. 1961, 36(6), 788-803.
- Silverman R.B. The Organic Chemistry of Drug Design and Drug Action, Second. ed., Elsevier Academic Press. (2004)
- Liu, J. Y.; Yu, H. L.; Quan, Z. S.; .Piao, H. R. Bioorg. Med. Chem. Lett. 2009, 19, 2392-2935.
- Belloli, S.; Moresco, R.M.; Matarrese, M. Neurochem. Int. 2004, 44, 433– 440
- Wentland, M.P.; et al. Drug. Des. Discov. 1997, 15(1), 25-38
- Al-Bayati, R.I.H.; Radi, M.F. Afr. J. Pure. Appl. Chem. 2010, 4(10), 228-232
- Chan, T.H.; Wong, L.T.L. J Org Chem. 1969, 34(9), 2766-2767
- Andrews, J.M. J Antimicrob Chemother. 2001, 48(S1), 5-16
- Louis, K.S.; Siegel, A.C. Methods. Mol. Bio. 2011, 740, 7-12

This work is licensed under a Creative Commons Attribution 4.0 International License.









