Studies on the chemical constituents of leaves of Phyllanthus emblica (L.)
Jaya Gupta, Amit Gupta and A.K.Gupta
Department of chemistry, Agra College, Agra
DOI : http://dx.doi.org/10.13005/ojc/300474
Article Received on :
Article Accepted on :
Article Published : 17 Nov 2014
A Phytochemical study was carried out on the leaves of Phyllanthus emblica. By using different chromatographic techniques the separation of the chemical compounds were done and structure of the compounds were elucidated by spectroscopic methods including nuclear magnetic resonance as well as mass spectrometry. Two compounds were isolated and identified; that are quercetin and β-sitosterol.
KEYWORDS:Euphorbiaceae; Phyllanthus emblica; quercetin; β-sitosterol; NMR
Download this article as:| Copy the following to cite this article: Gupta J, Gupta A, Gupta A. K. Studies on the chemical constituents of leaves of Phyllanthus emblica (L.). Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Gupta J, Gupta A, Gupta A. K. Studies on the chemical constituents of leaves of Phyllanthus emblica (L.). Available from: http://www.orientjchem.org/?p=5410 |
INTRODUCTION
Phyllanthus emblica Linn., belongs to the family Euphorbiaceae, commonly known as Indian gooseberry and amla. It is distributed in tropical and subtropical regions of India. It is excellent source of vitamin C, easily assimilated by human body1.It is found all over India, along the sea-coast districts, in deciduous forest of Madhya Pradesh. It is helpful in lowering cholesterollevel2 and protects from heart disease3-4, strengthens senses5, strengthens liver6. It is useful in diabetes7-8, gonorrhea, diuretic fevers, diarrhoea9, mouth ulcers, inflammations, hair growth, headache, colic, asthma, respiratory problems. It is used as antioxidant10, aphrodisiac, antifungal, antiviral, anticancer, antigenotoxic11, antimutagenic12, chelating agent.
EXPERIMENTAL
1H NMR and 13C NMR spectra were recorded on a Bruker Advance 400 MHz spectrometer. The EI-mass was recorded on Shimadju QP 2000 mass spectrometer. The leaves of Phyllanthus emblica was collected from Agra College, Agra. The leaves were air dried under shade for ten days. Then the leaves were powdered with the help of warming blender. The air dried powdered leaves (500gm) were subjected to successive hot extraction in a Soxhlet apparatus with solvents petroleum ether, ethyl alcohol and ethyl acetate. The average time period for extraction was 72 hours. The individual extracts were filtered twice and then concentrated by distillation on vaccum. The ethanolic extract (5gm) was subjected to silica gel chromatography using isopropanol-formic acid-water (2:5:5), to give compound 1 quercetin.
A portion of the ethyl acetate extract (4 gm) was subjected to silica gel thin layer chromatography using n-hexane: acetone (80:20) solvent system to give compound 2 that is identified β-sitosterol. Quercetin and β-sitosterol were identified by comparison with data from previous NMR and mass spectra 13-15.
Quercetin: Compound (1) Slightly yellow powder; m.p 316 °C; 1H NMR (400 MHz, Me OD): δ (ppm) = 6.20 (1H, d, J = 2.0 Hz, H-6), 6.42 (1H, d, J = 2.0 Hz, H-8), 6.90 (1H,d, J = 8.2 Hz, H-5’), 7.64 (1H, dd, J = 8.3; 2.1 Hz, H-6’), 7.76 (1H, d, J = 2.1 Hz, H-2’),13C NMR (100MHz, Me OD): δ (ppm) = 148.4 ( C-2), 137.1 ( C-3), 177.4 ( C-4),162.4(C-5), 99.2 ( C-6), 165.6 ( C-7), 94.8 ( C-8), 158.2 ( C-9), 104.6 ( C-10), 124.5 ( C-1’), 116.2 ( C-2’, C-5’), 146.4 ( C-3’), 150.2 ( C-4’), 121.7 ( C-6’).
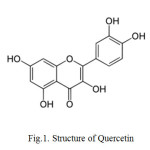 |
Fig1: Structure of QuercetinClick here to View figure |
β-Sitosterol : Compound (2) White powder, m.p1360C, 1HNMR (CDCl3,400MHz): 5.38(1H,dd,J=5.2Hz,H-6),3.56(1H,tt,J=11.3;5.3Hz,H-3), 2.34 (1H,ddd,J=13.0;5.0;2.0Hz,H-4a) 0.74,0.87,0.88,0.89,0.93,1.06(each3H,s,MeX6). 13CNMR(CDCl3,100MHz):δ37.1(C-1), 31.5 (C-2),71.7(C-3),42.2(C-4),140.6(C-5),121.6(C-6), 31.8 (C-7),31.7(C-8),50.2(C-9),36.4(C-10), 21.2(C-11),39.7(C-12),42.2(C-13),56.7(C-14),24.2(C-15),28.1(C-16),56.0(C-17), 11.7(C-18), 19.3(C-19), 36.3 (C-20), 18.9(C-21), 33.8(C-22),26.1(C-23), 45.7(C-24), 29.2(C-25),19.7(C-26),19.1(C27),23.0 (C-28),12.1(C-29).
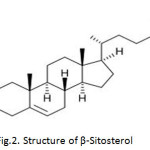 |
Fig2: Structure of β-Sitosterol Click here to View figure |
Results and Discussion
Aim of this study was to identify and characterize the bioactive principles from the leaves of Phyllanthus emblica. Compound 1 is a slightly yellow powder, m.p 316 °C. The EI-mass spectrum of 1 showed the molecular ion at m/z 302 [M+] corresponding to the formula C15H10O7 and in agreement with other spectroscopic data. The 1H NMR showed that the proton of H-6 and H-8 appeared as a duplet at δ 6.20 and 6.42 .The proton of H-5’,H-6’and H-2’ appears at δ 6.90,7.64 and 7.76 respectively.13CNMR spectrum showed a carbonyl group at δ177.4 .The carbon bonded to hydroxyl group appeared at δ137.1,146.4,150.2,162.4 and 165.6.
Compound 2 was isolated as white powder, m.p 1360C. The EI-mass spectrum of 2 showed the molecular ion at m/z 414[M+] corresponding to the molecular formula C29H50O and in agreement with other spectroscopic data. The 1H NMR spectrum showed a broad triplet at δ 5.38 corresponding to H-6 olefenic proton and multiple at δ 3.56 corresponding to H-3 alpha proton six tertiary methyl singlets.13C NMR of the compound showed 29 signals for steroid skeleton which was represented by six methyl groups. The carbon bonded to the hydroxyl group C-3 appeared at 71.7.The quercetin and β- sitosterol has been earlier reported in various plants 16-20.
CONCLUSION
From the physical, chemical and spectral characteristics, compound 1and 2 were concluded as Quercetin (Fig.1) and β- sitosterol (Fig.2). Quercetin is flavonoid, used in asthma, eczema, heyfever, and hives. It possess anti-inflammatory, anticancer, antiviral activity, inhibit inflammatory leukotriene production21. β- sitosterol is phytosterol, used as antioxidant and an anti- diabetic agent. Human liver microsome studies reveals that β- sitosterol inhibits the cholesterol absorption, reduces the symptoms of benign prostatic hyperplasia22, anti-inflammatory23 and anti-pyretic activity. So medicinal properties of Phyllanthus emblica is due to the quercetin and β- sitosterol, furthermore scientific evaluation are required to establish therapeutic efficacy.
ACKNOWLEDGEMENT
We are very grateful to University Grants Commission, New Delhi, India for their financial assistance (Grant No.F.15-39/12 (SA-II)). We are also very thankful to Dr.M.K.Rawat, Principal Agra College, Agra for their support.
REFERENCES
- Nisha,P.;Singhal.R.S., and Pandit, A.B.; Int JFood Sci Nutr.2004,55(5),415-422.
- Kim, H.J.;Yokozawat,Kimhy.,Tohda,C.;Rao,T.P.; and Juneja,L.R.; J Nutr Sci Vitaminol.2005,51,413-418
- Yokozawa,T.;Kim,H.Y.;Kim,H.J.;Okubo,T.;Chu,D.C.; and Juneja,L.R.;Br.J.Nutr.2007,97(6)1187-1195
- Mathur,R.;Sharma,A.;Dixit,V.P.; and Verma,M.;J Ethnopharmacol,1996,50(2),61-68
- Reddy,V.D.;Padmavathi,P.V.;Kavitha,G.;Gopi,S.; and Varadacharyulu, N.,J.Med Food 2011, 14(1-2),62-68
- Tasduq,S.A;Kaisar,P.; and Gupta,D.K.;PhytoetherRes.2005,19(3),193-197
- Nampoothiri,S.V.;Prathapan,A.;Cherian,O.C.;Raghu,K.G.;Venugopalan,U.V.; and Sundaresan,A.;Food ChemToxicol,2011,49(1),125-131
- Babu,P.S.;Stainley,Mainzen,; and Prince,P.,J.Pharm.Pharmacol,2004,56,(11),1435-1442
- Mehmood,M.H.;Siddiqi,H.S.; and Gilani,A.H.; J.Ethnopharmacol,2011,133(2),856-865
- Naik,G.H.; and Priyadarsini,K.I.;Phytoether Res.2005,19(7),582-586
- Banu,S.M.;Selevendiran,K.;Singh,J.P.; and Sakthisekaran,D.; Hum.Exp.Toxicol,2004,23,527-531
- Madhavi, D.; Rudrama,Devi.K.; Desava,Rao.K.; and Reddy,P.P.; J.Envi.Biol.2007,28,115-117
- Praveen, S.; Kader, Md.A.; Muhit, Md.A; Haque, Md.E.; and Mosaddik, Md.A.; JAPS,2011 1(9) 47-50
- Karan,S.K.;Mishra,S.K.;Pal,D.K.; and Mandal,A.;J.Med.Plant Res.,2012,6(7),1219-1223
- Trivedi, P.C.; and Chaudhary,N.; JPR, 2011, 4(11) ,4252-4253
- Rahman,S.M.M.;Mukta,Z.A.; and Hossain,M.A.; As J Food Ag-Ind,2009, 2(1),39-43
- Akhtar,P.; Ali,M.; Sharma, M.P.;Farooqi,H.; and Khan, H.N.; J.Phytol, 2010,2(3), 89-100
- Khanam,S.; and Sultana,R.; IJSPR 2012,3(4), 1057-1060
- Sarin,R., and Bansal,N.; IJRAP 2011,2(3),927-930
- Subramaniam,S.S.; and Nair,A.G.R.;Phytyochem,1972,11,439
- Numata.Y.; and Tanaka,H.;Food Chemistry,126,2011,751-755
- Wilt,T.J.;Macdonald,R.;and Ishani,A.; B.J.U Int., 1999,83(9),976
- Loizou.S.,Lekakis,I.; Chrousos,G.P.;and Moutsatou,P.,Mol.Nut.Food Res.,2010,54,551.

This work is licensed under a Creative Commons Attribution 4.0 International License.









