Specification of the Operational Parameters Contribution in the Efficiency of TiO2-P25 Nanoparticles in the Photocatalytic Removal of Cr(VI) by Taguchi Method
Maryam Sabonian and Mohammad A. Behnajady*
Department of Chemistry, College of Science, Tabriz Branch, Islamic Azad University, Tabriz, Iran
DOI : http://dx.doi.org/10.13005/ojc/300463
Article Received on :
Article Accepted on :
Article Published : 18 Nov 2014
Chromium exists in environment both as trivalent [Cr(III)] and hexavalent [Cr(VI)] forms. However, hexavalent form is five hundred times more toxic than the trivalent form. Heterogeneous photocatalysis processes, using aqueous suspensions of semiconductors, have received considerable attention in the removal of toxic metals from aqueous media. In this work the nanoparticles of TiO2-P25 in the form of slurry were used for photoreduction of Cr(VI) to the less harmful Cr(III). The process has been conducted in different operational conditions such as different initial concentrations of Cr(VI), dosage of photocatalyst, irradiation times, irradiation intensities of light and pH. For the optimization of the process Taguchi experimental design was used. The results of optimization using the Taguchi method, indicated that the pH with 28%, initial concentration of Cr(VI) with 26.99% and dosage of TiO2 nanocatalyst with 20.53% have the most effects among the selected factors. The intensity of UV light irradiation has the least effect on the efficiency of the process.
KEYWORDS:Photocatalytic removal; Optimization; Titanium dioxide nanoparticles; Taguchi method; Cr(VI)
Download this article as:| Copy the following to cite this article: Sabonian M, Behnajady M. A. Specification of the Operational Parameters Contribution in the Efficiency of TiO2-P25 Nanoparticles in the Photocatalytic Removal of Cr(VI) by Taguchi Method. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Sabonian M, Behnajady M. A. Specification of the Operational Parameters Contribution in the Efficiency of TiO2-P25 Nanoparticles in the Photocatalytic Removal of Cr(VI) by Taguchi Method. Available from: http://www.orientjchem.org/?p=5456 |
INTRODUCTION
Cr(VI) is one of the heavy metals which is a very toxic pollutant found in industrial wastes1. It is widely used in many industries, such as stainless and alloy steels2, pigments, tanning agents, catalysts3, petroleum refining process, electroplating and corrosion-resistant products4,5. The two oxidation states of chromium, Cr(III) and Cr(VI) have extremely different environmental and chemical properties6,7. Cr(VI) is toxic for human beings, animals, plants and microorganisms8. Hexavalent state is 500 times more toxic than trivalent state and the toxic effects of Cr(VI) on human beings include skin irritation, lung cancer, and harmful effects on kidneys, liver and gastric9. Cr(VI) can be removed from aqueous waste by a variety of techniques, such as chemical precipitation, reverse osmosis8, ion exchange, foam floatation, electrolysis and adsorption. Most of these methods require high amount of energy or a large amount of chemical substances; therefore, the process of photocatalysis is more effective than the other techniques in this regard5,10. In aqueous solutions, the most important chemical species of Cr(VI) in terms of the total concentration and pH are: HCrO4–, CrO42- and Cr2O72- 11. TiO2 has proved to be a promising photocatalyst because of its high photocatalytic activity, non-toxic nature, good stability, and low-cost fabrication12. Photocatalytic method is based on the reactive properties of the electron-hole pairs generated in the semiconductor particles under illumination by light whose energy is greater than the semiconductor band gap5,13. These charge carriers can reach the surface of the particles and react with the species in solutions with suitable redox potential1,14.
Jiiang et al studied Cr(VI) photoreduction with TiO2 and sulfated TiO2 3. Yang et al studied photocatalytic reduction of Cr(VI) in aqueous solution with ZnO under the visible light15. Iwata et al studied photocatalytic reduction of Cr(VI) on TiO2 film formed using anodizing16. Schrank et al studied the reduction of Cr(VI) and dye oxidation in TiO2 slurry reactor17.Doménech and Muñoz studied the photocatalytic reduction of Cr(VI) on ZnO powder13.
The degree of the effect that each influential factmor has on the characteristics of the output can be expressed in the form of an equation by an experimental design. The goals of experimental design in this study include reducing the number of experiments, lowering costs and determining the variables with the most effect on response 18,19. The removal of unnecessary factors, calculating the percentage of importance of each variable, determining the extent of error and determining the optimum conditions are among other goals of the experimental design. The Taguchi method was developed by Genichi Taguchi between 1950 and 1960 to improve the implementation of total quality control in Japan20. Taguchi used orthogonal arrays to reduce the number of the experiments considerably21. These arrays are selected out of the total number of experiments with special features.
In the present study, the removal of Cr(VI) was done using UV/TiO2 process, and to optimization the mentioned process and determine the share of each parameter, Taguchi’s experimental design was utilized.
Material and methods
Materials
Potassium dichromate, nitric acid and sodium hydroxide were purchased from Merck (Germany). TiO2-P25 was Degussa and it constitutes approximately 80% anatase and 20% rutile. It had a BET surface area of 50 ± 15 m2 g-1 and an average particle diameter of 21 nm, containing 99.5% TiO2. The TEM image of the TiO2-P25 nanoparticles has been show in Fig. 1. TEM image evidenced a wide heterogeneity in the size of the titania particles, ranging from ca. 10 to 50 nm.
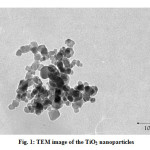 |
Fig1: TEM image of the TiO2 nano particles |
Photoreactor and procedure
In this work, slurry nanoparticles of TiO2-P25 was utilized. First, a suspension of TiO2-P25 nanoparticles the pH (HANNA pH 211, Romania) of which had been regulated was put under ultrasonic waves (Elma T460/H) in order to increase the dispersion of TiO2 in water. Then, the obtained suspension was put inside a quartz tubular photoreactor equipped with a UV lamp (15 W, UV-C, λmax= 254 nm, manufactured by Philips, Holland) vertically placed in front of the reactor. Following this, the suspension was transferred into the reactor. The reaction cell was bubbled with O2 at a flow rate of 0.5 mL min–1 in the darkness for 15 min before irradiation. At given irradiation time intervals, the samples (5 mL) were taken out, centrifuged (Hettich EBA) and then Cr(VI) concentration was analyzed by UV–Vis spectrophotometer (Pharmacia Biotech, Ultrospec 2000) at λmax= 350 nm (Chakrabarti et al. 2009).
Taguchi method
Taguchi’s method is a combination of statistical and mathematical techniques in experimental studies which is used in the design and analysis of experiments22,23. The most important Taguchi design is fractional factorial design which is used for estimating the effects of many factors with two or more levels on the response using orthogonal array24,25. The analysis of the experimental data using ANOVA estimate the effect of the factors on the characteristics properties19.
RESULT AND DISCUSSION
In order to obtain maximum information for optimization of Cr(VI) photocatalytic removal using TiO2-P25 nanoparticles, Taguchi’s experimental design was used. In this method, the features of orthogonal arrays were used to obtain the number of experiments needed. In this part, five variables, including initial concentration of Cr(VI), the dosage of TiO2 catalyst, light irradiation time, the intensity of UV irradiation and the solution pH were considered as main factors affecting upon photocatalytic activity. Five variables change at two levels. The factors and levels used in this work are initial concentration of Cr(VI)(20 and 40 mg L-1), dosage of TiO2 nanocatalyst (100 and 250 mg L-1), light irradiation time (15 and 30 min), intensity of UV light irradiation (18.25 and 33.26 W m-2) and pH (2 and 4). If factorial design is used, for 25 experiments, a total of 32 experiments are needed. It is clear that full factorial design needs more experiments. Therefore, in this method, with 3 repetitions of the 8 experiments, we will have a total of 24 experiments that will help us understand the extent of the effect of the mentioned parameters.
By considering the five factors in 2 levels of change for each one, suitable orthogonal array should be selected. Since five factors have a degree of freedom of 1, total degrees of freedom would be 5. Therefore, L8 would be the suitable array. Table 1 shows the L8 array mentioned above. In this array 5 factors change at 2 levels. Each line of the matrix indicates one experiment. According to Taguchi’s orthogonal array L8, eight experiments were used to evaluate the effect of five variables on the efficiency of Cr(VI) removal. For ensuring lower probability of error, each experiment was repeated three times.
For determining the optimum conditions and the share of each parameter involved, the methods of averaging and drawing graphs were used. The values of average are shown in Table 1. In Fig. 2, the average response for each parameter is displayed. The results based on the average indicate that the optimum conditions for the initial concentration of Cr(VI) is level 1 (20 mg L-1), the dosage of TiO2 nano catalyst is level 2 (250 mg L-1), the time of light irradiation is level 2 (30 min), the intensity of UV irradiation is level 2 (33.26 W m-2) and the solution pH is level 1 (2). Because, in this study, the performance statistics of ”the bigger – the better” was used to define the optimum conditions.
Table 1: L8 orthogonal array and average response
|
Average response |
Factors levels |
Number of experiments |
||||
|
pH |
Intensity of UV irradiation (W m-2) |
Light irradiation time (min)4 |
Dosage of TiO2 nanocatalyst (mg L-1) |
Initial concentration of Cr(VI) (mg L-1) |
||
|
29.41 |
1 |
1 |
1 |
1 |
1 |
1 |
|
69.12 |
1 |
2 |
2 |
2 |
1 |
2 |
|
19.83 |
1 |
2 |
2 |
1 |
2 |
3 |
|
26.58 |
1 |
1 |
1 |
2 |
2 |
4 |
|
21.24 |
2 |
2 |
1 |
1 |
1 |
5 |
|
24.51 |
2 |
1 |
2 |
2 |
1 |
6 |
|
5.96 |
2 |
1 |
2 |
1 |
2 |
7 |
|
19.55 |
2 |
2 |
1 |
2 |
2 |
8 |
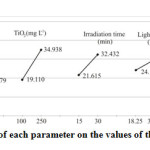 |
Fig2: The effect of each parameter on the values of the average response Click here to View figure |
In Taguchi’s method, analysis of variance (ANOVA) was used for determining the effect of each input parameters and the share of each of factors. The results of ANOVA are reported in Table 2. As shown in this table, solution pH with 28%, initial concentration of Cr(VI) with 26.99% and dosage of TiO2 nano catalyst with 20.53% have the most effects among the selected factors. The light irradiation time with 9.284% has the fourth rank in affecting the efficiency of Cr(VI) photo catalytic removal, and the intensity of UV light irradiation has the least effect on the efficiency of the process.
Table 2: The results of ANOVA for determining the percentage of the effect of different factors
|
Percent P(%) |
Pure sum (S’) |
F-Ratio (F) |
Variance (V) |
Sum of squares (S) |
DOF (f) |
Factors |
|
26.99 |
1923.02 |
48.52 |
1963.49 |
1963.49 |
1 |
Initial concentration of Cr(VI) (mg L-1) |
|
20.53 |
1462.75 |
37.14 |
1503.21 |
1503.21 |
1 |
Dosage of TiO2 nanocatalyst (mg L-1) |
|
9.28 |
661.53 |
17.35 |
702.00 |
702.00 |
1 |
Light irradiation time (min) |
|
2.13 |
151.74 |
4.75 |
192.21 |
192.21 |
1 |
Intensity of UV irradiation (W m-2) |
|
28 |
1994.94 |
50.30 |
2035.41 |
2035.41 |
1 |
Solution pH |
CONCLUSIONS
Taguchi’s designing method with L8 array was used for determining the share of each operational parameter for Cr(VI) photocatalytic removal using TiO2 nanoparticles. The Taguchi’s experimental design methodology shows that among the effective parameters on Cr(VI) photocatalytic removal efficiency, the greater effect is related to pH. The results revealed that the optimal conditions for the photocatalytic removal of Cr(VI) are initial concentration of Cr(VI) at level 1 (20 mg L-1), dosage of TiO2 nano catalyst at level 2 (250 mg L-1), time of light irradiation at level 2 (30 min), intensity of UV irradiation at level 2 (33.26 W m-2) and solution pH at level 1 (2). This indicates that Taguchi’s experimental design is a fast, reliable and efficient way of determining the optimum conditions of the process.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge for the financial support of Tabriz Branch, Islamic Azad University and the Iranian Nanotechnology Initiative Council.
REFERENCES
- Colón, G.; Hidalgo, M.C.; Navı́o, J.A. J. Photochem. Photobiol. A. 2001, 138, 79-85.
- Fournier-Salaün, M.C.; Salaün, P. Cent. Eur. J. Chem. 2007, 5, 1084-1093.
- Jiang, F.; Zheng, Z.; Xu, Z.; Zheng, S.; Guo, Z.; Chen, L. J. Hazard. Mater. 2006, 134, 94- 103.
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Ray, A.K.; Dutta, B.K. Chem. Eng. J. 2009, 153, 86- 93.
- Rengaraj, S.; Venkataraj, S.; Yeon, J.W.; Kim, Y.; Li, X.Z.; Pang, G.K.H. Appl. Catal. B. 2007, 77, 157-165.
- Lin, S.H.; Chen, C.N.; Juang, R.S. J. Environ. Manage. 2009, 90, 1950-1955.
- Golder, A.K.; Chanda, A.K.; Samanta, A.N.; Ray, S. Sep. Purif. Technol. 2011, 76, 345-350.
- Ölmez, T. J. Hazard. Mater. 2009, 162, 1371-1378.
- Gupta, V.K.; Rastogi, A.; Nayak, A. J. Colloid Interface Sci. 2010, 342, 135-141.
- Mohapatra, P.; Samantaray, S.K.; Parida, K. J. Photochem. Photobiol. A. 2005, 170, 189-194.
- Wang, S.-L.; Chen, C.-C.; Tzou, Y.-M.; Hsu, C.-L.; Chen, J.-H.; Lin, C.-F. J. Hazard. Mater. 2009, 164, 223-228.
- Wang, Q.; Shang, J.; Zhu, T.; Zhao, F. J. Mol. Catal. A: Chem. 2011, 335, 242-247.
- Domenech, X.; Muñoz, J. J. Chem. Tech. Biotechnol. 1990, 47, 101-107.
- Khalil, L.B.; Mourad, W.E.; Rophael, M.W. Appl. Catal. B. 1998, 17, 267-273.
- Yang, G.C.C.; Chan, S.-W. J. Nanopart. Res. 2009, 11, 221-230.
- Iwata, T.; Ishikawa, M.; Ichino, R.; Okido, M. Surf. Coat. Technol. 2003, 169–170, 703-706.
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M. J. Photochem. Photobiol. A. 2002, 147, 71-76.
- Zolfaghari, G.; Esmaili-Sari, A.; Anbia, M.; Younesi, H.; Amirmahmoodi, S.; Ghafari-Nazari, A. J. Hazard. Mater. 2011, 192, 1046-1055.
- Kaminari, N.M.S.; Schultz, D.R.; Ponte, M.J.J.S.; Ponte, H.A.; Marino, C.E.B.; Neto, A.C. Chem. Eng. J. 2007, 126, 139-146.
- Mousavi, S.M.; Yaghmaei, S.; Jafari, A.; Vossoughi, M.; Ghobadi, Z. Chem. Eng. Process. 2007, 46, 935-940.
- Aber, S.; Khataee, A.; Sheydaei, M. Bioresour. Technol. 2009, 100, 6586-6591.
- Engin, A. B.; Özdemir, Ö.; Turan, M.; Turan, A.Z. J. Hazard. Mater. 2008, 159, 348-353.
- Kim, K.D.; Choi, D.W.; Choa, Y.-H.; Kim, H.T. Colloids Surf. A. 2007, 311, 170-173.
- Wang, J.; Wan, W. Int. J. Hydrogen Energy, 2009, 34, 235-244.
- Beril Gönder, Z.; Kaya, Y.; Vergili, I.; Barlas, H. Sep. Purif. Technol. 2010, 70, 265-273.

This work is licensed under a Creative Commons Attribution 4.0 International License.









