Comparison of physio-chemical characterization of Ceiba Pentandra wood waste and Ipomea Carnia Stem waste by H3Po4 treatment for the dye removal
Geetha, K*1, N. Velmani2, S. Karthikeyan3 and Syed Shabudeen4
*1Department of Chemistry, Kalaignar Karunanidhini Institute of Technology , Coimbatore-641043,India.
2Department of Chemistry, Government Arts College, Coimbatore-641007, India.
3Department of Chemistry, Chikkana Government Arts College, Tirupur. India.
4Department of Chemistry, Kumaraguru college of Engineering and Technology, Coimbatore. India
DOI : http://dx.doi.org/10.13005/ojc/300466
Article Received on :
Article Accepted on :
Article Published : 15 Nov 2014
Ceiba Pentradenta wood waste and Ipomea Carnia stem waste has been activated using phosphoric acid and shows excellent improvement in their surface characteristic. Adsorption properties along with surface functional groups in surface morphology play a significant role. Micropores, mesopores and macropores are seen in the activated carbon. The resulting samples were characterized by nitrogen adsorption measurements at 77 K to obtain surface area and pore size distributions.SEM analysis has been carried out to determine its pore structure and the Fourier transformation infrared spectroscopy techniques was used for the investigation of surface functional groups. Physico-Chemical characteristics such as bulk density, moisture content, ash content, matter soluble in water, matter soluble in acid, pH, conductivity, porosity, pHzpc, decolourising power and surface area have been carried out to assess the suitability of the carbon as absorbent. Ceiba Pentradenta wood waste and Ipomea Carnia stem waste showed a good result by H3PO4 impregnation process followed by activation at 800 °C under nitrogen atmosphere.
KEYWORDS:Carbonization; Ceiba Pentradenta wood waste; Ipomea Carnia stem waste; Phosphoric acid treatment; physio-chemical characteristic
Download this article as:| Copy the following to cite this article: Geetha K, Velmani N, Karthikeyan S, Syed Shabudeen S. Comparison of physio-chemical characterization of Ceiba Pentandra wood waste and Ipomea Carnia Stem waste by H3Po4 treatment for the dye removal. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Geetha K, Velmani N, Karthikeyan S, Syed Shabudeen S. Comparison of physio-chemical characterization of Ceiba Pentandra wood waste and Ipomea Carnia Stem waste by H3Po4 treatment for the dye removal. Orient J Chem 2014;30(4). Available from: http://www.orientjchem.org/?p=5376 |
Introduction
Dye is the first pollutant to be recognized in wastewater1. The presence of very small amounts of dyes in water is highly visible and undesirable1,2. The decolonization of waste water is a major ecological alarm. Synthetic dyes have been broadly excreted in the waste water from different manufacturing, particularly from textile, paper, rubber, lather, cosmetic, food and drug industries which used dyes to color their products. It is reported that over 100000 commercially available dyes exist and the worldwide annual production of synthetic dyes is more then 7x 105 metric tons 3. Colored waste matter from dye industries give unwanted perspective to the water streams where as some dyes and their metabolites pose toxic, carcinogenic, mutagenic and teratogenic effects.
The removal of the dye from effluent using adsorption process provides an attractive alternative treatment, especially if the adsorbent is readily available and inexpensive. Agricultural waste by product have been converted into activated carbons possessing high specific porosity, high surface areas and are extremely resourceful adsorbents of major industrial significance. These activated carbons are widely used for the removal of effluent dye which may be in form of a range of situation. There are various precursor materials such as those obtained from wood, agricultural waste, coal etc., A number of non conventional low cost adsorbent used for the dye removal are as follows; Prosopis Juliflora 4, wood 5, waste Orange Peel 6, Banana pith7-8, coir pith9, Bagasse Pith 10-11, maize cob 12, barley husk 13,apple pomace14, and melon seed husk15.India is an agricultural based economy and more than 200 million ton of farming waste is generated every year. The use of agricultural waste can create employment and the pollution created due to this throwaway material can be prohibited. For the past few decades, attention has been shifted towards adsorption technique, which emerged as one of the widely accepted methods for the removal of contaminants from wastewater. One of the major challenges associated with adsorption using activated carbon is its cost effectiveness. Researchers in the recent past have mainly focused on preparation of activated from agricultural waste materials as an alternative for the commercial activated carbon.
Research directed towards surface chemistry of activated carbon with their performance on the adsorption of the dye16 has been referred in literature. It has been found that the precursor with surface properties and specific functionalities was found to undergo an excellent adsorption of adsorbate in its solution. The activated carbon with sufficient amount of super microporosity and mesoporosity possess enhanced characteristics for adsorption of dyes. This work presents the comparisons between characterizations of two agricultural waste products which cannot be used even for fireworks, as the wood is found to be soft in nature. These precursors are normally exposed to a number of different activation methods such as physical or chemical in an effort to achieve carbon with high adsorption capacity for a particular application. In this present investigation the activated carbon derived from Ceiba Pentradenta bark and Ipomea Carnia Stem waste with various techniques such as Scanning Electron Microscopy (SEM) and Fourier Transformation Infrared Spectroscopy (FT-IR) in order to understand the properties. The intention of this paper is to study the physico-chemical characteristics of activated carbon prepared from agricultural waste wood by various physical and chemical activation process with a view to use them in the treatment of wastewater.
Experimental methods and procedure
The physico –Chemical characteristic for the agricultural waste product such as Ceiba Pentradenta bark waste and Ipomea Carnia Stem waste were analyzed. The bark and stem were collected from in and around area of Erode. The wood and stem were cut into small pieces of 2 to 3 cm in size, dried in the sun light for 10 to 20 days to remove moisture from the material. Then the dried material was used for preparation of activated carbons using physical and chemical activation methods. The precursor underwent various carbonization treatments using calcium chloride, sodium sulphate, sulphuric acid, sodium carbonate and Phosphoric acid, of which carbonization using phosphoric acid gave a better result. The procedure has been explained below,
Carbonization using Phosphoric acid (17)
The precursor material to be carbonized was soaked with phosphoric acid in the ratio of 1:1 at 80 0C for 48 hours. After 48 hours the material was crushed well using mortar and this crushed material was kept aside for 12 hours. Further the material was washed well with hot water until a neutral pH was obtained, and dried at 110 0C for 24 hour. The dried mass, subjected to carbonization process at 800 0C for about 10 minutes was again thermal activated at 400 0C for about 10 minutes in the presence of nitrogen atmosphere. The final product was grounded well and used for subsequence analytical assessment.
Characterization of Activated carbon
The moisture content (%) by mass, ash content (%) by mass, Carbon, Nitrogen, Oxygen by %. Ion exchange, Bulk density, Matter soluble in water, Matter soluble in acid, Surface area, pHzpc were calculated according to reported procedures of standard analytical techniques. pH and conductivity measurement were analyzed using Elico make pH meter (model L1 -120) and conductivity meter (model M-180), respectively were performed as per standard procedures.
Surface area and Pore size distribution Analysis
The N2 adsorption-desorption isotherms of activated carbon were measured at 77K using N2 gas sorption analyzer (Nova 1000, Quanta Chrome Corporation) in order to determine the surface area and total pore volume. The surface area was calculated using the BET equation. In addition, the t-plot method applied to calculate the micropore volume and external surface area (Mesoporous Surface area). The total pore volume estimated using liquid volume of adsorbate (N2) at a relative pressure of 0.99. All the surface area calculated from the nitrogen adsorption isotherms by assuming the area of a nitrogen molecule was 0.162 nm2.
FT-IR spectra and SEM
The electronic structure of carbon samples were examined using FT-IR 1725 x (Perkin-Elmer) spectrometer. The measurements were carried out over the range 4000-400 cm-1. Carbon samples (0.33 wt%) were stirred with dry KBr (Merck, spectroscopy grade) and then pressed to form appropriate tablets. The surface morphology of carbon samples were observed with SEM (HITACHI S3000N).
Table1: Activated carbon prepared from Ceiba pentradenta wood waste and Ipomea Carnia Stem waste by carbonization with Phosphoric acid
| SAMPLE |
TREATMENT PROCESS |
||||
| ACP | H3PO4 Process + Thermal Activation under N2 flow | ||||
| AIC | H3PO4 Process + Thermal Activation under N2 flow | ||||
* ACP –Activated carbon from Ceiba Pentradenta soft wood waste
* AIC –Activated carbon from Ipomea Carnia stem waste
Table 2 : Physio chemical properties of Activated Carbons prepared by Ceiba pentradenta wood waste and Ipomea Carnia Stem waste
|
S.No |
PROPERTY |
ACTIVATED CARBON | |
|
ACP |
AIC |
||
|
1. |
pH |
4.3 |
|
|
2. |
Moisture Content (%) |
4.4 |
7.2 |
|
3. |
Ash Content (%) |
12 |
10 |
|
4. |
Conductivity ms/cm |
0.88 |
0.58 |
|
5. |
Specific Gravity (S) |
1.29 |
1.1 |
|
6. |
Bulk Density (D) |
0.3248 |
0.3421 |
|
7. |
Porosity (%) |
74.8 |
68.9 |
|
8. |
Matter soluble in Water (%) |
1.04 |
1.90 |
|
9. |
Matter soluble in acid (%) |
2.21 |
2.70 |
|
10. |
Carbon (%) |
68.09 |
72.01 |
|
11. |
Hydrogen (%) |
5.27 |
7 |
|
12. |
Nitrogen (%) |
0.11 |
0.24 |
|
13. |
Sulphur (%) |
ND |
ND |
|
14. |
Oxygen (%) |
26.53 |
18.40 |
|
15. |
Ion exchange capacity mg/l |
0.52 |
0.64 |
|
16. |
Decolorizing Power mg/l |
39 |
42 |
|
17.
|
BET Surface Area (m2/g) |
377.65 |
440 |
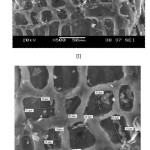 |
Fig1: SEM Photograph of (I & II) ACP |
 |
Fig2: SEM Photograph of (III & IV) AIC Click here to View figure |
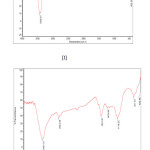 |
Fig3: FT-IR spectra of (I) ACP, (II) Click here to View figure |
 |
Fig4: XRD pattern for AIC Click here to View figure |
 |
Figure 5 Click here to View figure |
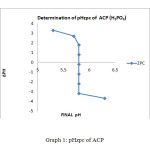 |
Graph1: pHzpc of ACP
|
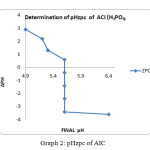 |
Graph2: pHzpc of AIC |
Result and Discussions
From the table 2 the moisture content of both the activated carbon are found to be moderate and they show extensive porosity in the carbon structure. The ash content of both the carbon was found to be comparatively less. This attributed to lower inorganic content and higher fixed carbon. The surface area of the precursor plays a vital role in the dye adsorption, higher the surface area prepared by the phosphoric acid process may be due to the restricted pore shrinkage during activation. Carbons with high surface area considered are the most superior for adsorption of organic substances18. Activated carbon shows significant nitrogen uptake at low pressure. That can be attributed to the strong interaction between nitrogen molecules and the wall with closely spaced pores. Nitrogen adsorption for the highest surface area obtained for activated carbon prepared using Ceiba Pentradenta wood waste by Phosphoric acid process followed by activation at 800 °C under a nitrogen atmosphere (377 m2g-1). Similar procedure had been adopted for Ipomea Carnia Stem the surface area was 440 m2g-1.
FT-IR spectrum of Ceiba Pentradenta wood waste and Ipomea Carnia Stem waste prepared by Phosphoric acid processes shown in the fig 3(I&II) revealed that they contain four classes of surface oxides such as carboxyls, lactones, phenols and carbonyls. The concentration of the surface groups varied, depending on the activation conditions. The assignment of the specific wave number to a given functional group was not possible because the adsorption bands of various functional groups overlap and shift depending on their molecular structure and environment. Shifts in absorption position may be caused by factors such as intramolecular and intermolecular hydrogen bonding, steric effect and degree of conjugation. For instance, within its given range, the position of C=0 stretching band (common to carbonyls, carboxylic acids and lactones) is determined by many factors, such as the physical state, electronic and mass effects of neighbouring substituent’s, conjugation, hydrogen bonding and ring strain19. The functional group with respect to the oxygen was likely to be affected by some or all of the factors listed above. Most of the carbons exhibit similar IR spectroscopic features; those are very intense/sharp -OH stretching of carboxyl, phenol and alcohol vibration around 1054 cm-1 and aliphatic CH stretching absorption around 2900 cm-1. Saturated aliphatic ethers show a strong band in the region 1064.69 cm-1 was attributed to carbonyl groups, and broad band in the region 1500 to 1900 cm-1 due to C=0 stretching. The group of bands appeared in the region 2778.46 cm-1 corresponding to –CH2 groups. The broad band observed in the region of 1000 to 1250 cm-1 was assigned due to a characteristic absorption of -OH group. These results are in good agreement with the findings of many investigators20.
The morphological study by SEM of the above adsorbents shown in the Figurers 1& 2 exhibited a highly porous material. From the SEM results, it was found that there are holes and cave type openings on the surface of the specimen that would definitely have increased the surface that are available for the adsorption21.
The ZPC for the ACP showed that a graph has been plotted between ∆pH and Final pH. The value of ∆pH decreases gradually from 3.3 to -3.7 and the final pH value vary from 5.3 to 6.3, but a final pH value of 5.8 is maintained between the pH of 4 to 9.The graphical representation for ZPC is given below for ACP method. The similar trend has been observed for the AIC ∆pH decreases gradually from 3to -4 and the final pH has been maintained at 5.4. It has shown in the graph.
Activated carbon used in the dye removal
The acid, reactive and direct dyes have anionic character and basic dyes are cationic character. Figure 6 shows the percentage of anionic and cationic dye removal for ACP and AIC vs pH . A secure connection between surface area and surface groups of the customized activated carbons and percentage of dye removal through adsorption can be observed. Activated carbons prepared from wood waste carbon and stem waste a similar behavior was observed for anionic dyes (i.e reactive, direct and acid dyes) following an increase in the adsorption capacity with increase in the surface area.
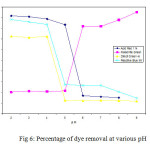 |
Fig6: Percentage of dye removal at various pHClick here to View figure |
References
- Banat, I. M.; Nigam, P.; Singh, D.; Marchant., R .; Microbial decolorization of textile-dye-containing effluents: A review. Bioresource Technology, 1996, 58:217–227.
- Robinson, T.; McMullan, G.; Marchant., R..; Nigam, P.; Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative, Bioresource Technology, 2001, 77:247–255.
- Pearce, C. I.; Lloyd, J.R.; and Guthrie, J.T.; The removal of colour from textile wastewater using whole bacterial cells: A review, Dyes and Pigments, 2003, 58: 179–196.
- Somasekhara, M.C.; Treatment of hazardous dye house waste water by adsorption using prosopis Juliflora fruit waste, Proc Int Conf Environ & Bioetheics, 2000, 264-279.
- Asfour, H.M.; Fadali, O.A.; Nassar, M,M.; & El-Fuendi, M.S.; Equilibrium studies on adsorption on basic dyes on hard wood ,J Chem Technol Biotechnol, 1985, 35 A, 21-27.
- Namasivayam, C.; Muniaswamy, N.; Gayathri, K.; Rani, M.; & Ranganathan, K.; Removal of dyes from aqueous solution by cellulosic waste orange peel, Biores. Technol, 1996, 57, 37-43.
- Namasivayam, C.; Kanchana , N.; & Yamuna , R.T.; Waste banana pith as adsorbent for the removal of Rhodamine-B from aqueous solution, Waste Manage , 1993,13 , 89-95.
- Namasivayam, C.; Radhika, R.; & Suba,S.; Uptake of dyes by a promising locally available agricultural solid waste: coirpith, Waste Manage, 2001 , 21, 381-387.
- Mckay, G.; El-Geundi, M,S., & Nassar, M,M., Equilibrium studies during the removal of dyestuffs from aqueous solution using bagasse pith, Wat Res,1978, 2 , 1513-1520.
- Laszlo,J,A., Removing acid dyes from textile waste water using biomass for decolourization ,Am. Dyestuff Rep, 1994, 17-21.
- El-Geundi, M,S., Color removal from textile effluent by adsorption techniques, Wat Res, 1991,25 , 271-273.
- Robinson,T.;Chandran,B.; Sathya Naidu,G.; Nigam,B.; Studies on the removal of dyes from synthetic textile effluent using barley husk in static-batch mode and in a continuous flow, packed-bed,reactor, Biores Technol, 2002,85, 43-49.
- Robinson,T.; Chandran,B.; Nigam,B.; Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomance and wheat straw, Wat Res, 2002,36 ,2824-2830.
- Gang Sun &Xu , XiangjingSunflower stalks as adsorbents for color removal from textile wastewater, Ind Eng Chem Res, 1997, 6 , 808-812.
- Okieman, F.C.; &.Ohyenkpa V.V.; Bio waste, 1989,29,11.
- Manuel Fernando, R.; Pereira Samant,F.; SoaresJose, J.M.O.; Kfao Jose,L.; Figureueriredo,A., Carbon, 2003, 41,811.
- Nasser,M.M.; El-Geundi,M.S.; J Chem Biotechnol , 1991,50, 257.
- Hu , Z.; Srinivasan,M.P.; In: 5th International Activated Carbon Technology Board of Singapore (NSTB) under a grant for Conference, the Pittsburgh Plaza, Pittsburgh, USA, 1997.
- Kendall, D.; Applied Infrared Spectroscopy, Reinhold Publishing Corp. Chapman & Hall Ltd, London, 1996,560.
- Zawndski,J.; Carbon, 1981,19, 19.
- Khattri,S.D.; Singh, M.K.; J. Chem. Technol., 1999, 6, 116 .

This work is licensed under a Creative Commons Attribution 4.0 International License.









