Synthesis of Fe3O4 nanoparticles from Ironstone from the Republic of Yemen.
Nabil Abdullah Noman Alkadasi
Department of Chemistry ,Faculty of Education and Science ,Rada’a , Al-baida'a ,University , Yemen
DOI : http://dx.doi.org/10.13005/ojc/300330
Article Received on :
Article Accepted on :
Article Published : 26 Sep 2014
In this study, a new preparation of Fe3O4 nano particles is reported. Fe3O4 nano particle were successfully synthesized . This method consisted of two stages, beginning with the pulverization and separation of iron ore from ironstone by using the coprecipitation method of magnetite. The characterization of Fe3O4 nano particle was done by TEM ,XRD and U.V.
KEYWORDS:Magantite; Fe3O4 nano particles and properties and characterization
Download this article as:| Copy the following to cite this article: Alkadasi N. A. N. Synthesis of Fe3O4 nano particles from Ironstone from The Republic of Yemen. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Alkadasi N. A. N. Synthesis of Fe3O4 nano particles from Ironstone from The Republic of Yemen. Orient J Chem 2014;30(3). Available from: "ttp://www.orientjchem.org/?p=5110 |
Introduction
Recently, a considerable amount of research focused on iron oxides , due to their potential uses in pigments, drug deliverg and resonance imaging for clinical diagnosis, recording material and catalyst, etc[ 1-3]. The magnetic nano particles exhibit superparamagnetic behavior because of the infinitely small coercivity arising from the negligible energy barrier in the hysteresis of the magnetization loop of the particles as predicted [ 4]. There are many ways to prepare Fe3O4 nano particles, which have been reported in other papers, such as arc discharge, mechanical grinding, laser ablation, microemulsions, and high temperature decomposition of organic precursors, etc. These methods are used to prepare magnetice nanoparticle with controlled diameters. However, well-dispersed aqueous Fe3O4 nano particles have been met with very limited success. Several methods have been published for synthesizing Fe3O4 nano particles, and several research studies have reported the successful preparation of nano- or microscale Fe3O4. Using different methods, such as the ultrasonic chemical coprecipitation method and the solvothermal method [2-6] have been for reported the synthesis of nano particle Fe3O4 in organic solvent, and Cupper [ 7] successfully fabricated magnetic Fe3O4 covered with a modifiable phospholipid coating . Of these methods, chemical coprecipitation was reported to be the most promising because of its simplicity and productivity [8-10]. The physics of nano scale magnetic materials has been a vivid subject for researchers within the last few decades and the exploration of iron sand from beaches or rivers to prepare magnetic materials on nano scale has been reported in some studies [11]. In this paper, magnetic materials from ironstone mining in Pasaman Barat West Sumatera were investigated, and it was found that ironstone in that area contained 12.462 ppm of iron (Fe), with a susceptibility magnetic value of 888.81 x 10-8 m3/kg by using an atomic absorption spectro photometer and magnetic susceptibility meter.For these reasons, these materials have the potential to be developed and cultivated as raw materials for magnetite (Fe3O4). Although there have been many significant developments in the synthesis of magnetic nano particles, the stability of these particles without agglomeration or precipitation is an important issue. It began with the crushing of ironstone into powder form and then synthesizing Fe3O4 nano particles by using the coprecipitation method of magnetite ore.
Experiment
Materials
Hydrochloric Acid ( HCl ) and Ammonia Solution ( NH4OH ) were purchased from Sinopharm chemical reagent Co ,Ltd ,China,and ironstone was obtained from Republic of Yemen.
Physical parameters of Hydrochloric Acid ( HCl ) , Ammonia Solution ( NH4OH ) and Fe3O4 powder are reported in table 1 , 2 and 3 respectively.
Table 1. General Characteristics of Hydrochloric Acid ( HCl )
| Molecular formula | Hydrochloric Acid ( HCl ) |
| Appearance | liquid |
| Molecular weight | 36 .5 |
| Concentration | 36 – 38 % |
| Company | Sinopharm chemical reagent Co ,Ltd ,China |
Table 2. General Characteristics of Ammonia Solution ( NH4OH )
| Molecular formula | Ammonia ( NH4OH ) |
| Appearance | liquid |
| Molecular weight | 17.03 |
| Concentration | 25 – 28 % |
| Company | Sinopharm chemical reagent Co ,Ltd ,China |
Table 3. General Characteristics of Fe3O4 powder
| Molecular formula | Magentite oxide powder ( Fe2O3 ) |
| Appearance | Brown powder |
| Fe3O4 % | 45.31-75 |
| Chorite | 37.73-13 |
| Riebeckite | 16.95-15 |
| Country | Al-Baida’a ,Yemen. |
 |
Photo1: Fe3O4 Rock Fe3O4 in powder form Click here to View Photo |
Experiment
Two steps of prepararing samples have been reported here . In the first step ironstone was pulverized to obtain a powder . Then a permanent magnet was used to obtain the iron ore.In the second step the iron ore powders were prepared by the chemical coprecitation method .
In typical coprecipitation synthesis procedure , 10 g Fe3O4 powder and 20 ml HCl were mixed and heated at 90 0C for one hour . The solutions were filtered and then 25 ml NH4OH (90%) was added to the filtrate .The black precipitate was collected and washed with de-ionized water and pure ethanol three times.
 |
Photo.2 : Equipment Fe3O4 Nano particle ( after dry ) Click here to View Photo |
Transmission Electron Microscope ( TEM ) Test
For TEM Test , a small amount of sample was dissolved in 3mL of deionized water in test tube and the solution was stirred by ultra-sonication . Then 10 µ L sample was transferred to clean Copper Grid and kept for drying for TEM test.The TEM micro graphs of samples were observed by CM 12 Philips Transmission Electron Microscope .
 |
Fig1: XRD for Fe3O4 Iron PowderClick here to View Figure |
 |
Fig2: Fe3O4 nano particle Click here to View Figure |
Results and Discussion
The Fe3O4 nano particle was synthesized by heating to 90 0C of Fe3O4 powder . plate 1,2,3 ,4,5,6and 7 ( TEM ) shows the top-view TEM images of the Fe3O4 Nano particle plate ( TEM ) 1 The size of the Fe3O4 nano particle is clear from the TEM. The surface of Fe3O4 nano particle shows several large meandering wrinkles. The size of Fe3O4 nanoparticle can be clear from TEM image . Fig (1and 2) X-ray differaction shown the graph all of Magnitite and Fe3O4 nano particle. Fig (3and 4) U.V shown the graph all of Magnitite and Fe3O4 nano particle respectively dispersed in chloroform.
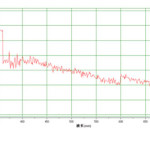 |
Fig3: Magnetite Click here to View figure |
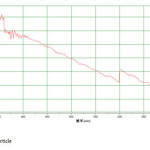 |
Fig4: Fe3O4 nano particle Click here to View Figure |
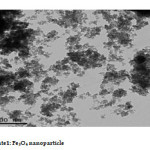 |
Plate1: Fe3O4 nano particle Click here to View Plate |
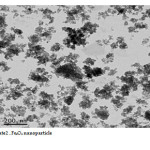 |
Plate2: Fe3O4 nano particleClick here to View Plate |
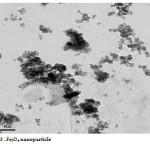 |
Plate3: Fe3O4 nano particleClick here to View Plate |
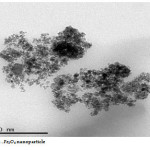 |
Plate4: Fe3O4 nano particle Click here to View Plate |
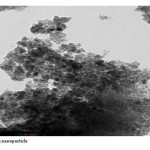 |
Plate5: Fe3O4 nano particle Click here to View Plate |
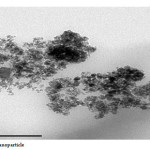 |
Plate6: Fe3O4 nano particle Click here to View Plate |
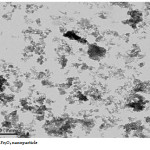 |
Plate7: Fe3O4 nano particleClick here to View Plate |
References
- Astuti, G.Claudia, Noraida, and M.Ramadhani, Makara J. Sci, August 2013 | Vol. 17 | No. 2.
- P. Tartaj, M.P. Morales, S.V-Verdaguer, T. GCarreno,C.J. Serna, J. Phys. D: Appl. Phys. 36 (2003) R182.
- S. Wu, S. Aizhi, Z. Fuqiang, J. Wang, X. Wenhuan, Z. Qian, A.A. Volinsky. J. Mater. Lett. 65 (2011) 1882.
- S. Bedanta. Dissertation, Universitat Duisburg, Essens, 2006.
- A. Yan, X. Liu, G. Qiu, H. Wu, R. Yi, N. Zhang, J. Xu, J. Alloy. Compd. 458/1-2 (2008) 487.
- Z. Yuanbi, Q. Zumin, H. Jiaying, Chinese J. Chem.Eng. 16/3 (2008) 451.
- H.T. Hai, H. Kura, M. Takahashi, T. Ogawa. J. Colloid Interface Sci. 341 (2010) 194.
- M.D. Cuyper, P. Müller, H. Lueken, M. Hodenius, J. Phys: Condens. Matter. 15 (2003) S1425.
- H.E. Ghandoor, H.M. Zidan, Mostafa, M.H. Khalil, M.I.M. Ismail, Int. J. Electrochem. Sci. 7 (2012) 5734.
- S. Mendoza, R.A. Morales, L. Flores, J.P. Hinestroza, V.S. Mendieta. J. Nanopart. Res. 14 (2012) 1242.
- W. Jiang, H.C. Yang, S.Y. Yang, H.E. Horng, J.C. Hung, Y.C. Chen, C.Y. Hong, J. Magn. Magn. Mater. 283 (2004) 210.
- T. Rusianto, M.W. Wildan, K. Abraha, Kusmono, J. Teknologi. 5/1 (2012) 62.
- P. Hu, S. Zhang, H. Wang, D. Pan, J. Tian, Z. Tang, A.A. Volinsky, J. Alloy. Compd. 509 (2011) 2316.
- Y. Hou, Z. Xu, S. Sun, Angew. Chem. Int. Ed. 46 (2007) 6329.
- S.Y. Zhao, D.K. Lee, C.W. Kim, H.G. Cha, Y.H. Kim, Y.S. Kang, Korean Chem. Soc. 27/2 (2006) 237.
- C.R. Lin, T.C. Tsai, M. Chung, S.Z. Lu. J. Appl. Phys. 105 (2009) 07B509.
- O. Rahman, S.C. Mohapatra, S. Ahmad, J. Mater. Chem. Phys. 132 (2012) 196.
- L.L. Wang, J.S. Jiang, Nanoscale Res. Lett. 4 (2009) 1439.
- S.P. Gubin, Y.A. Koksharov, G.B. Khomutov, G.Y. Yurkov, Russ. Chem. Rev. 74/6 (2005) 489.
- R. Nowosielski, J. Achievements Mater. Manuf. Eng. 24/1 (2007) 68.
- F. Schuth, A.H. Lu, E.L. Salabas, Angew. Chem. Int. Ed. 46/8 (2007) 1222.
- J.S. Val, J. González, In: A. Méndez-Vilas, J. Díaz (Eds.), Microscopy: Science,Technology,Applications and Education, Microscopy Book Series no.4, vol. 3, Formatex Research Center, Badajoz, Spain,2010,1620.

This work is licensed under a Creative Commons Attribution 4.0 International License.









