Synthesis of 1,3,4-Thiadiazoles, α -Pyranone ,Pyridine, Polysubstituted Benzene from 1,3,4-Thiadiazolyl Ethanone and Testing Against Tuberculosis Based on Molecular Docking Studies
Mahmoud. M. Abdelall1,2
1Chemistry Department, Faculty of Science, Al-Azhar University, Nasr City11884, Cairo, Egypt. 2Chemistry Department, Faculty of Science and Art, Al-Baha University, Al-Baha, Saudi Arabia.
DOI : http://dx.doi.org/10.13005/ojc/300321
Article Received on :
Article Accepted on :
Article Published : 22 Aug 2014
The synthesis is reported of new 1,3,4-thiadiazole, imidazopyridine, α-pyranone, pyrazolo[1,5-a]pyrimidine, polysubstituted benzene, pyridine , pyrazoles and pyrazolo[3,4-d]pyridazine using 1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}ethanone as starting material.
KEYWORDS:1,3,4-thiadiazole; enaminone; dithioester; polysubstituted benzen
Download this article as:| Copy the following to cite this article: Abdelall M. M. Synthesis of 1,3,4-Thiadiazoles, α -Pyranone ,Pyridine, Polysubstituted Benzene from 1,3,4-Thiadiazolyl Ethanone and Testing Against Tuberculosis Based on Molecular Docking Studies. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Abdelall M. M. Synthesis of 1,3,4-Thiadiazoles, α -Pyranone ,Pyridine, Polysubstituted Benzene from 1,3,4-Thiadiazolyl Ethanone and Testing Against Tuberculosis Based on Molecular Docking Studies. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4283 |
Introduction
1,3,4-Thiadiazoles have been screened for their antibacterial and antifungal activities1-4, anti-inflammatory5,anti-tuberculosis activity6.The development of efficient methods for the synthesis of 1,3,4-thiadiazoles has attracted significant interest. Substituted 1,3,4-thiadiazoles have become very useful compounds in medicine , agriculture, and in many fields of technology such as dyes7,lubricating compositions8, optically active liquid crystals9, photographic materials10, and many others. Also, applications of 1,3,4- thiadiazoles in agriculture as herbicides11,fungicides12, and bacteriocides13 have been patented. Also, 1,3,4-thiadiazole derivatives have shown anti-inflammatory activity14-16.
Results and Discussion
Chemistry
The synthetic strategies adopted to obtain the target compound are depicted in Schemes1. The key intermediate 1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}ethanone (3) was prepared in an excellent yield by condensing methyl (4-methoxyphenyl)carbamodithioate(1) with (1E)-2-oxo-N-phenylpropanehydrazonoyl chloride (2). the structure of 3 was established on the basis of analytical and spectral data. The 1HNMR spectrum showed signals at 2.61(s,3H,COCH3) 3.81 (s,3H ,OCH3) 6.91 , 6.97 (dd, 4H, Ar-H AB-system ) 7.36 – 8.01 (m,5H,ArH).
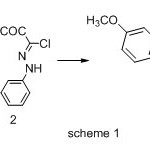 |
Scheme 1 Click here to View Scheme |
Treatment of 1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}ethanone (3) with methyl/ or benzyl hydrazinecarbodithioate17,18 in ethanol at reflux condenser afforded the corresponding dithioesters (4a,b).The 1HNMR spectrum of 4a showed signals at δ = 2.37(s, 3H, CH3-C=N), 2.62 (s, 3H, SCH3),3.73(s,3H,OCH3) ,7.16 – 7.97 (9H, m, Ar-H), 9.99 ppm (s, 1H, NH).while 1HNMR spectrum of 4b showed signals at δ=2.41(s, 3H, CH3-C=N), 3.77(s,3H,OCH3),4.53 (s, 2H, SCH2),7.10-7.93(14H, m, Ar-H),9.93 ppm(s, 1H, NH).
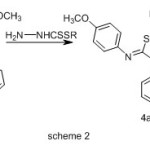 |
Scheme 2 Click here to View Scheme |
Interaction of methyl dithioester (4a) with hydrazonoyl halides (5a,b)19,20 in ethanol containing triethylamine at reflux temperature gave bis -1,3,4-thiadiazoles (8a,b). The structure of 8 was deduced from their spectral data and elemental analysis, Scheme (3). The 1HNMR spectrum of 8a showed signals at δ=2.32(s, 3H, CH3CO), 2.49 (s, 3H, CH3-C=N) , 3.78(s,3H,OCH3) ,7.02 – 7.99 ppm (14H, m, Ar-H) .Similarly, benzyldithioester (4b) reacted with the former hydrazonyl halides (5a,b) and produced products which were found to be identical in all respects (m.p, mixed m.p and spectra) with (8a,b). The formation of (8) can be explained via elimination of methyl mercaptan (or benzyl mercaptan) from the corresponding cycloadduct (7), which is assumed to be formed from 1,3-dipolar cycloaddition of nitrile imides (prepared in situ from 5 and triethylamine) to the C=S double bond in (4a) or (4b), (Scheme 3).
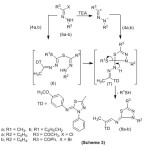 |
Scheme 3 Click here to View Scheme |
Enaminones are readily obtainable reagents and constitute an interesting class of compounds that are versatile precursors for the synthesis of several heterocyclic aromatic compounds. Thus, treatment of equimolar quantities of 1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}ethanone (3) with dimethylformamide-dimethylacetal (DMF-DMA) in refluxing xylene afforded the corresponding enaminone as (2E)-3-(dimethylamino)-1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}prop-2-en-1-one (9) (Scheme 4).
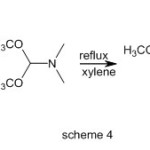 |
Scheme 4 Click here to View Scheme |
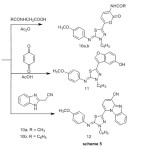
1H-NMR (δ ppm) spectrum (CDCl3) indicated signals at : 2.96 and 3.17 (2s, 6H, N(CH3)2) , 3.81 (s,3H,OCH3) , 5.90 and 7.85 (dd, 2H, olefinic CH=CH; J= 12 Hz), which support that the structure in (E-form), not (Z-form), 6.89 , 7.02 (dd, 4H, Ar-H AB-system ) 7.27 – 8.03 (m,5H,ArH).Enaminone (9) reacted with acetylglycine and benzoyl-glycine in acetic anhydride to give a products that were identified as N-(6-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}-2-oxo-2H-pyran-3-yl)acetamide and N-(6-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}-2-oxo-2H-pyran-3-yl)-benzamide (10a,b) respectively, which confirmed on the basis of elemental analysis and spectral data, (Scheme 5).
 |
Scheme 5 Click here to View Scheme |
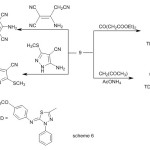
Interaction of enaminone (9) with 1,4-benzoquinone and 2-cyanomethylbenzimidazole afforded 11 and 12 respectively, (Scheme 5).condensation of enaminone (2) with diethyl acetonedicarboxylate in refluxing acetic acid afforded tetrasubstituted benzene derivative (13) (Scheme 6)1HNR of 13 showed signals at δ= 1.25 ,1.43 (2 t ,6H ,2CH2CH3) ,3.82(s ,3H ,OCH3) , 4.31, 4.44 (q , 4H ,2 CH2CH3 ) , 6.92-7.94 (m,11H, Ar-H) and 11.30ppm.(s , 1H, OH).
 |
Scheme 6 Click here to View Scheme |
Furthermore , reaction of enaminone 9 with other active methylene such as pentane-2,4-dione and 2-aminoprop-1-ene-1,1,3-tricarbonitrile afforded pyridine (14) and polysubstituted benzene (15) derivatives, respectively(Scheme 6).
Next, the reaction of enaminone 9 with heterocyclic amines was investigated. Refluxing of enaminone 9 with 5-amino-3-(methylsulfanyl)-1H-pyrazole-4-carbonitrile in glacial acetic acid gave 7-{(5E)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}-2-(methylsulfanyl)pyrazolo[1,5-a]-pyrimidine-3-carbonitrile (16) via elimination of water and dimethyl amine molecules (Scheme 6) .
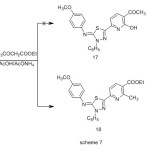
Condensation of enaminone 9 with ethyl 3-oxobutanoate were carried out in glacial acetic acid in the presence of ammonium acetate furnished a single product, for which the two possible structures 17 and 18 can be investigated (Scheme 7), but elemental analyses and spectral data were in complete accordance with the pyridinecarboxylate structure 18 .The IR spectrum of compound 18 revealed the absence of hydroxy band. Its 1H-NMR spectrum revealed a signals at δ 1.42 (t ,3H ,CH2CH3 ) and 4.41(q , 2H , CH2CH3) corresponding to ethyl ester protons , The structures of the products were confirmed by spectral (IR, MS and 1H-NMR) and elemental analyses (see Experimental).
 |
Scheme 7 Click here to View Scheme |
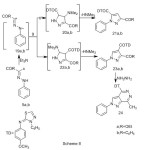
Finally, hydrazonyl halides 5a,b has been reported to add to α,β-unsaturated carbonyl compounds to yield a mixture of isomeric pyrazolines21,22. In the present work the reaction of enaminone (9) with nitrileimine (19a,b) (generated in situ from the treatment of the hydrazonoyl halide (5a,b) with triethylamine in refluxing m-xylene) gave only one isolable product (TLC). From which two proposed structures 21a,b or 23a,b seemed possible, (Scheme 8). 1H-NMR spectrum provided a firm support for structure 23 and ruled out the other possible structure 21. Thus, 1H-NMR spectrum of 23a and 23b exhibits a singlet at 8.52 and at 8.57 ppm , respectively which indicates the presence of pyrazole H-5 rather than H–423. Pyrazole derivative (23) was assumed to be formed via initial 1,3-dipolar cycloaddition of nitrileimine (19) to the activated double bond in compound 9 forming non isolable intermediate (22) followed by the loss of dimethylamine, (Scheme 8).Treatment of pyrazole 23a with hydrazine afforded pyrazolopyrdazine derivative 24 (Scheme 8).
 |
Scheme 8 Click here to View Scheme |
Molecular Modeling.
Docking studies
From analysis X-ray crystal structure of cytochrome P450 14α-sterol Demethylase (14DM) from Mycobacterium in complex with 4-phenylimidazole[24], through interaction with amino acid residues of (14DM), and demonstrated that, His-259 have a structural and functional evidence for the importance for inhibiting action of protein[24]. In order to obtained biological data on a structural basis, through rationalized ligand–protein interaction behavior, the molecular docking into the active site of (14DM) from Mycobacterium was performed for the synthesized compounds using flexible method. All calculations for docking experiment were preformed with MOE 2008.10 [24]. The tested compounds were evaluated in silico (docking), using X-ray crystal structures of (PDB entry code 1E9X) [24]) complexes with phenylimidazole. The tested compounds were docked into active sites of both enzymes (14DM). The active site of the enzyme was defined, to include residues within a 10.0 Å radius to any of the inhibitor atoms. The scoring functions were applied for the most stable docking model to evaluate the binding affinities of the inhibitors, which complexes with (14DM) active sites, table (1). The complexes were energy-minimized with an MMFF94 force field [26] until the gradient convergence 0.05 kcal/mol was reached. All isolated compounds were docked successfully, and compared with reference inhibitor (phenylimidazol 1), and exhibited that, the highest MOE binding scores for synthesized compounds 15< 16 < 4a < 4b Kcal/mol, respectively, (table 1).
The other parameters like hydrogen interaction energy, electrostatic interaction with the receptor, van der Waals and salvation energy were also taken into consideration for the evaluation of the docking results; the values of the energy and E-model were found to be significant, and accepted this fact that, a good van der Waals interactions decides the binding affinity for any ligand with receptor enzyme protein, and bad van der waals interactions shows ugly contacts through steric clashes after docking which should be less for good activity.
Structures activity relationships
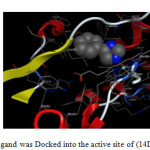 |
Fig1: The reference ligand was Docked into the active site of (14DM) using MOE tool. Click here to View Figure |
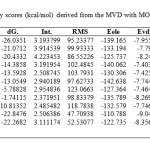 |
Table1: Docking energy scores (kcal/mol) derived from the MVD with MOE for isolated ligandsClick here to View table |
d.G.: free binding energy of the ligand from a given conformer, Int.: binding energy of hydrogen bond interaction with receptor, Eele: the electrostatic interaction with the receptor, Evdw: van der Waals energies between the ligand and the receptor, Esol.: Solvation energy.
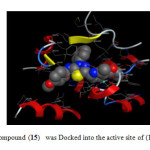 |
Fig2: The most active compound (15) was Docked into the active site of (14DM) using MOE tool. Click here to View Figure |
In order to get a deeper insight into the nature and type of interactions of docked compounds, the complexes between each compound and (14DM) receptor were visualized, and depicted in (Figs. 1 and 2). Since, the H bond interactions playing an important role in the structure and function of biological molecules, the current ligand-receptor interactions were analyzed on the basis of H bonding. In order to reduce the complexity, hydrophobic and π-cation interactions (>6Å) are not shown in (Figs. 1 and 2).
The results obtained clearly revealed that, the amino acid residues close to the reference molecules phenylimidazol (1) are mostly the same as observed in the currently compounds under investigation, which complexes with proteins . The higher binding energies and binding process interaction were observed in case of compounds 4a, 4b,15 and 16 act as inhibitors against (14DM).
Conclusion.
1,3,4-thiadiazolyl ethanone is a good starting material for the synthesis of different heterocyclic derivatives which gave a good results when tested against tuberculosis especially poly substituted benzene 15 ,pyrazolopyrimidine 16 and hydrazone 4 derivatives .
Experimental
All melting points and antimicrobial activities are uncorrected. IR spectra (KBr) were recorded on FT-IR 5300 spectrometer and Perkin Elmer spectrum RXIFT-IR system (ν, cm-1). The 1HNMR spectra were recorded in (CDCl3 & DMSO-d6) at (300) MHz on a Varian Mercury VX-300 NMR spectrometer (δ, ppm) using TMS as an internal standard. Mass spectra were obtained on GC Ms-QP 1000 EX mass spectrometer at 70 ev. Elemental analyses were carried out by the Micro analytical Research Center, Faculty of Science, Cairo University.
1-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}ethanone 3
A mixture of (1; 0.01 mol) and hydrazonoyl chloride 2 (0.01 mol) in ethanol (50 ml) triethylamine (0.5 ml) was added. The reaction mixture was refluxed for 3h. The resulting product was collected by filtration and recrystallized from EtOH to give 3 as yellow crystals .(yield 78%), m.p.130-132, ir(vcm-1) : 3076(CH-arom.) , 2958 (CH-aliph.) and 1678 (C=O), 1H NMR (300MHz δ ppm CDCl3) 2.61 (s, 3H, COCH3), 3.81 (s,3H,OCH3), 6.91 , 6.97(dd, 4H, Ar-H AB-system ) 7.39 (m, 1H, Ar-H),7.50(m,2H,Ar-H) and 8.01(d,2H,Ar-H). ms, m/z (intensity %) 325 (15.7).M.F. C17H15N3O2S. Calculated: C, 62.75, H, 4.65, N, 12.91. Found: C, 62.69, H, 4.60, N, 12. 87 .
Reaction of acetylthiadiazole with hydrazincarbodithioate
General procedure
A mixture of acetylthiadiazole (0.01 mol) and alkyl hydrazine carbodithioate (0.012 mol) in ethanol (50 ml) was refluxed for one hour. The separated solid on heating was filtered off and recrystallized from the proper solvent to give (4 a,b).
Methyl (E)-2-(1-((z)-5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethylidene)hydrazine-1-carbodithioate 4a
acetylthiadiazole (0.01 mol) and methyl hydrazine carbodithioate (0.012 mol) recrystallized from EtOH to give 4a as yellow crystals .(yield 84%), m.p.212-2140C, ir(vcm-1) :3159(NH), 3088(CH-arom.) and 2907 (CH-aliph.). 1H NMR (300MHz δ ppm CDCl3) 2.37 (s, 3H, CH3C=N), 2.62 (s,3H,SCH3)3.73 (s,3H,OCH3), 7.16-7.97 (m, 9H, Ar-H),and 9.99(s,1H,NH). M.F. C19H19N5OS3. Calculated: C, 53.12, H, 4.46, N, 16.30. Found: C, 53.09, H, 4.43, N, 16. 25 .
Benzyl (E)-2-(1-((Z)-5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethylidene)hydrazine-1-carbodithioate 4b
acetylthiadiazole (0.01 mol) and benzyl hydrazine carbodithioate (0.012 mol) recrystallized from EtOH to give 4b as yellow crystals .(yield 81%), m.p.207-2090C, ir(vcm-1) : 3174(NH), 3098(CH-arom.) and 2870 (CH-aliph.). 1H NMR (300MHz δ ppm CDCl3) 2.36 (s, 3H, CH3C=N), 2.61 (s,3H,SCH3)3.75 (s,3H,OCH3),4.53(s,2H,CH2) 7.10-7.93 (m, 14H, Ar-H),and 9.93(s,1H,NH). M.F. C25H23N5OS3. Calculated: C, 73.33, H, 5.66, N, 17.1. Found: C, 73.29, H, 5.59, N, 17. 04 .
Synthesis of 8 a,b
A mixture of (4a,b; 0.01 mol) and the appropriate hydrazonoyl halide (0.01 mol) in ethanol (50 ml) triethylamine (0.5 ml) was added .The reaction mixture was refluxed for 3h. The resulting product was collected by filtration and recrystallized from the proper solvent to give 8 .
1 1-((Z)-5-(((Z)-2-((Z)-5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)prop-1-en-1-yl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)ethan-1-one 8a
3 (0.01 mol) and(1E)-2-oxo-N-phenylpropanehydrazonoyl chloride (0.01 mol) recrystallized from benzene to give 8a as yellow crystals .(yield 76%), m.p.200-2020C, ir(vcm-1) : 3088(CH-arom.) , 2932
(CH-aliph.)and1685(CO). 1H NMR (300MHz δ ppm CDCl3) 2.32 (s, 3H, CH3C=N), 2.49 (s,3H,COCH3)3.81 (s,3H,OCH3),7.02-7.99 (m, 9H, Ar-H),and 9.93(s,1H,NH). M.F. C25H23N5OS3. Calculated: C, 73.33, H, 5.66, N, 17.1. Found: C, 73.29, H, 5.59, N, 17. 04 .
4.4.3 ((Z)-5-(((Z)-2-((Z)-5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)prop-1-en-1-yl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)(phenyl)methanone 8b.
3 (0.01 mol) and(1Z)-2-oxo-N,2-diphenylethanehydrazonoyl bromide (0.01 mol) recrystallized from benzene to give 8b as yellow crystals .(yield 75%), m.p.177-1790C, ir(vcm-1) : 3084(CH-arom.) 2931 (CH-aliph.)and1678(CO). 1H NMR (300MHz δ ppm CDCl3) 2.32 (s, 3H, CH3C=N), 3.74(s,3H,OCH3), 7.03-8.20 (m, 19H, Ar-H),and 9.93(s,1H,NH). M.F. C25H23N5OS3. Calculated: C, 73.33, H, 5.66, N, 17.1. Found: C, 73.29, H, 5.59, N, 17. 04 .
3 -(dimethylamino)-1-((Z)-5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)prop-2-en-1-one 9
A mixture of acetylthiadiazole (3; 0.01 mol) and DMF-DMA (0.012 mol) in dry benzene (40 ml) was heated under reflux for 5hrs. the separated solid was filtered off, washed with ethanol and recrystallized from benzene ,orange crystals .(yield 77%), m.p.165-1660C , ir(vcm-1) : 2924 (CH-aliph.) and 1662 (C=O), 1H NMR (300MHz δ ppm CDCl3) 2.96 and 3.17 (2s, 6H, N(CH3)2), 3.81(s,3H,OCH3)5.86 and 7.85 (dd, 2H, olefinic CH=CH; J= 12 Hz), 6.89 , 7.02(dd, 4H, Ar-H AB-system ) 7.31 (m, 1H, Ar-H),7.47(m,2H,Ar-H) and 8.02(d,2H,Ar-H),C13NMR(DMSO-d6): δ =175.01 ,155.77, 155.64, 154.55 , 150.38 , 144.62 , 139.01 , 128.74 , 126.65 , 122.94 , 121.37 , 120.77 , 120.71 , 114.78 , 88.23 , 55.16 , 44.78 , 40.1 and 39.55(2C). ms, m/z (intensity %) 293 (82.7).M.F. C13H12BrNO2. Calculated: C, 53.08, H, 4.11, N, 4.76. Found: C, 53.01, H, 4.08, N, 4.69.
Reaction of acylglycine with enaminone 9
A mixture of enaminone 9 (0.01 mol) and acetylglycine or benzoylglycine (0.01 mol) in acetic anhydride (30 ml) was heated under reflux for 2h. The reaction mixture was concentrated in vacuo. The solid product which formed upon cooling was filtered off then washed with ethanol and recrystallized from the appropriate solvents to give (10a,b).
1 N-(6-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}-2-oxo-2H-pyran-3-yl)acetamide 10a
Recrystallized from benzene. (yield 74%), m.p.242-2440C, ir (vcm-1): 3323 (NH), 1720 and 1673 (2C=O), 1H NMR (300MHz δ ppm DMSO-d6) 2.13 (s, 3H, CH3),3.74(s,3H,OCH3) , 6.91-7.51 (m, 9H, Ar-H), 7.94 and 8.20 (dd, 2H pyranone; J = 7.8 Hz), 9.85 (s, 1H, NH), C13NMR(DMSO-d6): δ =170.35,156.59,156.04,153.38,144.52,143.07,138.84,138.45,128.23, 126.60 ,122.41,121.33,114.89,106.59,55.19,40.37,39.81,38.70, M.F. C22H18N4O4S Calculated: C, 65.34, H, 4.98, N, 13.85. Found: C, 65.30, H, 4.95, N, 13.83.
N-(6-{(5Z)-5-[(4-methoxyphenyl)imino]-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl}-2-oxo-2H-pyran-3-yl)benzamide 10b
Recrystallized from benzene. (yield 81%), m.p.233-2350C, ir (vcm-1): 3386 (NH), 1719 and 1668(2C=O), 1H NMR (300MHz δ ppm DMSO-d6) 3.78(s,3H,OCH3), 6.95-7.85 (m, 9H, Ar-H),7.83 and 8.11 (dd, 2H pyranone; J=7.5 Hz), 9.90 (s, 1H, NH).ms, m/z (intensity %) 496 (32.9). M.F: C27H20N4O4S. Calculated: C, 69.82, H, 4.34, N, 12.06 Found: C, 69.78, H, 4.31, N, 12.02.
(Z)-3-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)benzofuran-5-ol (11).
To a stirred solution of enaminone 9 (0.01 mol) in glacial acetic acid (25 ml), 1,4-benzoquionone (0.01 mol) was added, stirring was continued for 3h. at room temperature. The reaction mixture was evaporated in vacuo and the solid product was isolated by filtration and recrystallized from acetic acid (yield 68%), m.p.189-1900C, ir (vcm-1): 3380 (OH); 1H NMR (300MHz δ ppm DMSO-d6) 3.76(s,3H,OCH3), 6.87-8.05 (m, 12H, Ar-H), 9.12 (s, 1H furan), 9.52 (s, 1H, OH). ms, m/z (intensity %) 399 (8.5). M.F: C23H17N3O3S. Calculated: C, 69.17, H, 4.29, N, 10.52 Found: C, 69.13, H, 4.26, N, 10.48.
(Z)-1-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)benzo[4,5] imidazo[1,2-a]pyridine-4-carbonitrile 12
A mixture of enaminone (9; 0.01 mol) and 2-cyanomethyl benzoimidazole (0.01 mol) in glacial acetic acid (30 ml) was refluxed for 3hrs. the solid product which obtained after cooling was collected by filtration and recrystallized from EtOH (yield 64%), m.p.148-1500C, ir (vcm-1): 2200 (CN); 1H NMR (300MHz δ ppm DMSO-d6) 3.77(s,3H,OCH3), 6.96-8.42 (m, 15H, Ar-H), m/z (intensity %) 426 (5.7). M.F: C27H18N6OS. Calculated: C, 76.04, H, 4.25, N, 19.71 Found: C, 76.01, H, 4.23, N, 19.68.
Diethyl (Z)-2-hydroxy-4-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)isophthalate 13
A mixture of enaminone (9; 0.01 mol) and diethyl acetonedicarboxylate (0.01 mol) in glacial acetic acid (30 ml) was refluxed for 2h. the solid product which obtained after cooling was collected by filtration and recrystallized from EtOH (yield 66%), m.p.170-1720C, ir (vcm-1): 1725 (CO); 1H NMR (300MHz δ ppm CDCl3)1.25(t,3H,CH2CH3) 1.43 (t,3H,CH2CH3), 3.82(s,3H,OCH3), 4.31(q,2H,CH2CH3), 4.44(q,2H,CH2CH3) , 6.92 – 7.94 (m, 11H, Ar-H),and 11.30 (s,1H,OH)M.F: C27H24N3O6S. Calculated: C, 66.66, H, 4.97, N, 8.64 Found: C, 66.61, H, 4.93, N, 8.63.
Ethyl (Z)-4-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)-2-methylbenzoate 14
To a solution of enaminone 9 (0.01 mol) and acetyl acetone (0.01 mol) in acetic acid (20 mL) was added ammonium acetate (0.02 mol). The reaction mixture was heated under reflux for 3 h. After cooling, the solid product was collected by filtration and crystallized from ethanol (yield71%) ; m.p.118-1200C, ir (vcm-1): 1677 (CO); 1H NMR (300MHz δ ppm CDCl3)2.62(s,3H,CH3) 2.73 (s,3H,COCH3), 3.82(s,3H,OCH3) and 6.94 – 8.06 (m, 11H, Ar-H); M.F: C23H18N4O2S. Calculated: C, 61.88, H, 4.06, N, 12.55 Found: C, 61.85, H, 4.03, N, 12.51.
(Z)-2,4-diamino-5-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carbonyl)isophthalonitrile 15
A mixture of enaminone 9 (0.01 mol) and 3-amino-2-cyanopent-2-enedinitrile (0.01 mol) in dioxan (25 mL) containing 1 ml of triethyl amine was heated under reflux for 3 h (followed until completion by TLC using 1:1 ethyl acetate–petroleum ether as eluent). The mixture was then cooled .The solid, so formed, was collected by filtration and crystallized from AcOH (yield 62%); m.p.280-2820C, ir (vcm-1): 3406,3331 (NH2) , 2211(CN) and1649(CO), 1H NMR (300MHz δ ppm DMSO-d6) 3.77(s,3H,OCH3),4.09(s,4H,2NH2) 6.96-7.89 (m, 10H, Ar-H); ms, m/z (intensity %) 576 (32.9); M.F: C24H17N7O2S. Calculated: C, 66.05, H, 4.16, N, 22.46 Found: C, 66.02, H, 4.12, N, 22.43.
(Z)-7-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)-2-(methylthio)pyrazolo[1,5-a]pyrimidine-3-carbonitrile 16
A mixture of enaminone 9 (0.01 mol) and 5-amino-3-(methylsulfanyl)-1H-pyrazole-4-carbonitrile (0.01 mol), in glacial acetic acid (20 mL), was refluxed for 3 h. The solid that formed was filtered off, and crystallized from ethanol to afford compounds 16 (yield 73%); m.p.170-1720C, ir (vcm-1): 2226(CN), 1H NMR (300MHz δ ppm CDCl3) 2.48(s,3H,SCH3) 3.80 (s,3H,OCH3) and 6.90-8.29 (m, 11H, Ar-H , pyrimidine-H); ms, m/z (intensity %) 407 (12.5) M.F: C23H17N7OS2 Calculated: C, 60.64, H, 3.76, N, 21.52 Found: C, 60.61, H, 3.73, N, 21.47.
Ethyl (Z)-6-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)-2-methylnicotinate 18
To a solution of enaminone 9 (0.01 mol) and ethyl 3-oxobutanoate (0.01 mol) in acetic acid (20 mL) was added ammonium acetate (0.0 2 mol). The reaction mixture was heated under reflux for 2 h. After cooling, the solid product was collected by filtration and crystallized from ethanol (yield 76%); m.p.130-1320C, ir (vcm-1): 1720(CO), 1H NMR (300MHz δ ppm CDCl3) 1.42(t,3H,CH2CH3), 2.81(s,3H,CH3), 3.83 (s,3H,OCH3),4.39(q,2H,CH2CH3), 6.92 , 7.02(dd, 4H, Ar-H AB-system;j = 8.4Hz) 7.33 (m, 1H, Ar-H),7.49(m,2H,Ar-H) 7.97(d,2H,Ar-H), (dd, 2H, pyridine-H;J = 8.4Hz); M.F: C24H20N4O3S Calculated: C, 60.49, H, 4.23, N, 11.76 Found: C, 60.46, H, 4.19, N, 11.73.
Synthesis of 23a,b
To a mixture of enaminone 9 (0.01 mol) and the hydrazonoyl halide 19a,b (0.01 mol) in benzene (30 ml) an equivalent amount of triethylamine was added. The reaction mixture was heated under reflux for 3h. the solvent was distilled at reduced pressure and the residual viscous liquid was taken in ethanol then the resulting solid was collected by filtration, washed thoroughly with ethanol, dried and finally crystallized from ethanol to give 23a,b.
Methyl (E)-4-(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazole-2-carbonyl)-1-phenyl-1H-pyrazole-3-carboxylate 23a
(yield 64%); m.p.144-1450C; ir (vcm-1): 1696 (CO), 1H NMR (300MHz δ ppm CDCl3) 2.68 (s, 3H, CH3), 3.82(s, 3H, OCH3), 6.91-8.08 (m, 14H, Ar-H), 8.52 (s, 1H, pyrazole-H5); calcd for C27H21N5O3S : C, 65.44 , H, 4.27, N, 14.13 Found: C, 65.40, H, 4.25, N, 14.09.
(E)-(3-benzoyl-1-phenyl-1H-pyrazol-4-yl)(5-((4-methoxyphenyl)imino)-4-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)methanone 23b
(yield 66%); m.p.148-1500C; ir (vcm-1): 1656 (CO) ; 1H NMR (300MHz δ ppm CDCl3) 3.80 (s, 3H, OCH3), 6.88 – 8.02 (m, 14H, Ar-H),8.58 (s, 1H, pyrazole-H5); calcd for C32H23N5O3S : C, 69.18, H, 3.81, N, 12.60. Found: C, 69.15, H, 3.78, N, 12.57
(E)-N-(4-methoxyphenyl)-5-(7-methyl-2-phenyl-2H-pyrazolo[3,4-d]pyridazin-4-yl)-3-phenyl-1,3,4-thiadiazol-2(3H)-imine 24
A mixture of pyrazole derivative 23a (0.01 mol) and hydrazine hydrate (0.012 mol) in ethanol (40 ml) was heated under reflux for 2h. The separated solid was filtered off, washed with ethanol and crystallized from ethanol (yield71%); m.p.248-2500C; ir (vcm-1): 1608 (C=N); 1H NMR (300MHz δ ppm DMSO-d6) 2.23(s,3H,CH3), 3.79(s,3H,OCH3), 6.72-7.81 (m, 14H, Ar-H), 8.43 (s, 1H, pyrazole-H5); ms: m/z (intensity %) 491 (74.4); calcd for C27H21N7OS: C, 61.94, H, 4.04, N, 18.73. Found: C, 61.91, H, 4.02, N, 18.69.
References
- kandapalli, V.G.; Vajja,S.R. Bull. Korean Chem. soc. 2010, 35, 1219-1222.
- Feray, A.; Cigdem, Y. Bull. Korean Chem. soc. 2001, 22, 476-480 .
- Dogan, H. N.; Rollas, S.; Erdeniz, H. , IL Farmaco 1998, 53, 462-467.
- Dogan, H. N. ; Duran A.; Rollas, S.; Sener, G.; Uysal, M. K.; Gulen D. Bioorg. Med.Chem. 2002 , 10, 2893-2898 .
- Palaska, E.; Sahin, G. ; Kelicen, P. ; Turlu, N. T. ; Altinok G. IL Farmaco 2002, 57,101-107.
- Karakus, S. ; Rollas, S. IL Farmaco 2002, 57, 577-581.
- Zareba, S. Pharmazie 1993, 48, 782–783.
- Gao, Y. J. ; Zhang, Z. J.; Xue, Q. J. Mater. Res. Bull. 1999, 34, 1867–1874.
- Choi, U. S.; Kim, T. W.; Jung, S. W. ; Kim C. J. Bull. Korean Chem. Soc. 1998, 19, 299–307.
- Chen, S. L.; Ji, S. X.; Zhu, Z. H. ; Yao Z. G. Dyes Pigments 1993, 23, 275–283.
- Jin, G. Y.; Hou, Z.; Zhao, G. F.; Cao, C. Y. ; Li Y. C. Chem. J. Chin. Univ. 1997, 18, 409–412.
- Lu, S. M.; Chen, R. Y. Org. Prep. Proced. Int. 2000, 32, 302–306.
- Hui, X. P.; Zhang, L. M.; Zhang, Z. Y.; Wang, Q. ; Wang, F. Indian J. Chem. Sect. B, 1999 , 38, 1066-1069 .
- Kumar, H.; Javed, S. A.; Khan, S. A. ; Amir, M. Eur. J. Med. Chem. 2008 , 43 , 2688–26983.
- Mullican, M. D.; Wilson, M. W.; Connor, D. T.; Kostlan, C. R.; Schrier, D. J. ; Dyer, R. D. J. Med. Chem. 1993, 36, 1090–1099.
- Song, Y.; Connor, D. T.; Sercel, A. D.; Sorenson, R. J.; Doubleday R.; Unangst P. C.; Roth B.D.; Beylin, V. G.; Gilbertsen, R. B.; Chan, K.; Schrier D. J.; Guglietta, A.; Bornemeier, D. A.; Dyer, R. D. J. Med. Chem. 1999, 42, 1161–1169.
- Danie,l L. K.; Joseph, F.; Scott, T. G.; Carl, J. M. ; John, P. S. J. Med. Chem., 1979 , 22, 855 -862.
- Korosi, J. Ger. Offen. 1, 934, 89929 Jan (1979), C. A. 72, 100334s (1970).
- Eweiss, N. F.; Abdelhamide, A.O. J. Heterocyclic. Chem. 1980 , 17, 1713 -1717 .
- Shawali, A. S.; Abdelhamide, A. O. Bull. Soc. Japan 1976 , 49, 321-324.
- Al-Zaydi, K.M. Molecules, 2003, 8 , 541-555.
- Helmut, B.;, Irmgard, B. ; Joachim, F. K. Arch. Pharm. (Weinheim) 1983 , 316 , 608-616.
- El-Taweel, F.M. ; Elnagdi, M. H. J. Heterocyclic Chem. 2001, 38 , 981-984.

This work is licensed under a Creative Commons Attribution 4.0 International License.









