Galfan I and Galfan II doped with calcium, Corrosion Resistant Alloys
Reza Amini Najafabadi*1, Missam Irani1, Izatulla Ganiev2, Ziadulla Obidov2
1Department of material science and engineering, Golpayegan University of Technology, Isfahan, Iran 2Chemistry Institute, Tajikistan Academy of Sciences, Dushanbe, Iran
DOI : http://dx.doi.org/10.13005/ojc/300307
Article Received on :
Article Accepted on :
Article Published : 02 Sep 2014
The results of electrochemical research on Zn5Al and Zn55Al alloys, doped with Calcium in the different concentration of natural ambient using Potentiodynamical method, indicate that, adding Calcium Ree due to move pitting and repassivation potentials to the positive area, and increasing concentration of chloride ions moves curves to the negative region. It is estimated that the small amount of Ca (0.005–0.05 wt %) reduce Corrosion rate of original alloy about 2-3 times. It means that the corrosion resistance of the Zn5Al and Zn55Al alloys improves considerably. Zn-Al-Ca alloy could be used as anode coatings to protect steel products and structures from corrosion.
KEYWORDS:Zn5Al; Zn55Al; corroson resistant; calcium; natural Ambient; NaCl
Download this article as:| Copy the following to cite this article: Najafabadi R. A, Irani M, Ganiev I, Obidov Z. Galfan I and Galfan II doped with calcium, Corrosion Resistant Alloys. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Najafabadi R. A, Irani M, Ganiev I, Obidov Z. Galfan I and Galfan II doped with calcium, Corrosion Resistant Alloys. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4529 |
Introduction
Aluminum is one of the most common elements on Earth’s surface. Due to its properties as low density and corrosion resistance alloys are widely used. Aluminum is a metal, whose production is growing fastest in the world, almost every area of the world needs it every once in a larger quantities. Since 1950, global consumption of aluminum increased to 17 times. In 2010 world production of aluminum was approximately 24 million tones1. Aluminum alloys and stainless steels are useful for engineers because of passive films of thin oxide .They arise naturally and reduce significantly the rate of corrosion of these alloys [1].
Zinc is the most widely used material for protection of steel against corrosion.The success of using zinc as a steel coating can be attributed to its sacrificial nature,low cost, and ease of application (hot-dipping or electroplating)[2].
longer life of construction materials are being increasingly required in order to promote resource conservation and reduce maintenance work. With regard to coated steel sheets, there is a limit in the increase of corrosion resistance of conventional Zn coated steel sheets. As a result, the more corrosion resistant Zn-Al alloy coated steel sheets are being used increasingly. In particular, since 55%Al-Zn and 5%Al-Zn alloy coated steel sheets exhibit excellent corrosion resistance, their use as a construction material for roofing and siding is rapidly expanding Eelectrochemical methods, especially potentiodynamic methods are powerful the chniques to investigate the corrosion protection of different coatings for substrate [3].
Gulf I is containing of 5 wt.% aluminum and zinc alloy and Gulf II is containig zinc alloy containing 55 weight percent aluminum are used as coatings for corrosion control of steel structures. Producing Gulf, galvalume and galvanized coatings, the only essential difference is handled mixed alloys in a bath of molten zinc[4] . The micro structure of these coatings, the so- called eutectic alloy has a composition that is solidified at a certain temperature, the eutectic temperature of the alloys [5].
Using potentiodynamic methods became possible to assess the role of the electrode potential of the alloy with passivation and passive condition [6]. It has turned out, that the dependence of the dissolution rate is essential feature of corrosion of metal which can be used to predict its corrosion resistance, and for choosing a method of protection at given conditions. In connection with the synthesis of new alloys and their introduction to the technique, as well as expanding the scope of application of zinc, aluminum and their alloys, especially in harsh environments, the issues of improving the corrosion resistance become important [7].
The present paper investigates the influences of calcium supplementation on the anodic behavior of Zn5Al (zinc alloy with 5wt.% Aluminum) and Zn55Al (Zinc alloy with 55 wt.% Aluminum) alloys, intended for the application of protective coatings hot method. (Should be continued)
Experimental Procedure
According to the standard of Ghost, the objects which were used were aluminum brand A7 grade, Zinc pure grade and calcium – KM1. These metal alloys were prepared in an alumina crucible in a shaft furnace type SSHOL resistance in the temperature range 750-8500С. The alloys were cast into a graphite mold rods, 8mm diameter and 140mm in length. Non-operating part of the samples was isolated resin (a mixture of 50% rosin and 50% paraffin). Before immersing the sample in the solution, its working end portion were cleaned by the sandpaper, degreased, etched in a 10 % solution of NaOH, washed thoroughly with alcohol and then immersed in a solution of NaCl to research. The temperature of the solution in the cell was maintained constant (20°C) using a thermostat MLSH 8. The silver chloride reference electrode which was served was the platinum subsidiary.
Anodic behavior of Zn5Al, Zn55Al alloys, doped with calcium conducted in 0.03%, 0.3% and 3 % concentrations of NaCl electrolyte by a potentiostat PI 50.1.1 with potential sweep rate 2mV/s by access to the programmer PR- 8 and recorder DTL -4 technique has been investigated [8].
When electrochemical potentiodynamic were studied, it was observed that samples were polarized in the positive direction of the potential steady after submerged, were polarized to a sharp increase in current due to pitting. Then the samples were polarized in the opposite direction to a potential of – 1,400 mV and were added the results in the alkalinization of the near-electrode layer of the alloy surface. Finally, the samples were re- polarized in the positive direction[9].
Calculation of the corrosion current as the main characteristics of the process of electrochemical corrosion, cathodic curve was carried out by taking into account bt = 0.12V taffy inclined in neutral environments with the process of pitting corrosion of aluminum and its alloys is controlled by the cathodic reaction of oxygen ionization[10].
Results and Discussion
Studied Chemical composition of the anode and the results of the behavior of the alloy Zn5Al, doped with calcium, are given in the Table 1. and Fig. 1 and 2.
Table 1 shows the results of the steady free capacity (fixed) corrosion time of Zn5Al alloy, calcium- doped at 0.03, 0.3 and 3% NaCl of electrolyte, which was recorded for one hour. Regardless of the chemical composition of all the investigated alloys, it is noted that potential shift in the positive region, which characterizes the dynamics of the formation of a protective oxide film, that is completed by 30-45 minutes from the dive and little depends on the chemical composition of the alloys. Thus, after one hour soaking in a solution of 0.3 % sodium chloride, the free corrosion potential of Zn5Al alloy is: – 1.070V , while the doped alloy containing 0.3 wt . % Calcium is: – 1.055V.
Results of electrochemical corrosion studies of aluminum-
zinc alloys doped with calcium, are presented in Table 1, indicate that calcium supplements in small amounts (0.005-0.05 wt. %) shifted stationary (free) corrosion potential of alloy Zn5Al in a positive area. However, further increase in calcium content in the alloys within 0.05 ¸ 0.3 wt. % shifted on the negative side and the behavior of alloys containing calcium has an extreme character (Fig. 1). A similar trend is occurring in all studied environments.
According to the Table 1, increasing concentrations of chloride ions, the potentials of free corrosion and the pitting corrosion rate of Zn5Al alloy, doped with calcium decreases, indicating a decrease in corrosion resistance of alloys exposed to chlorine ions. Doped calcium alloys are characterized by a lower value of corrosion rate than the original alloy. Established relationship is consistent with a change in the corrosion rate of aluminum – zinc alloys with different concentrations of calcium (Table 1).
As an example, Fig. 2 shows the anodic polarization Potentiodynamic curves of zinc-aluminum alloy doped with calcium, an electrolyte ambient in 3% aqueous NaCl, showing that all of the curves with 0.005-0.3% calcium supplements are arranged in the region of positive values of the potential compared to the curve 1 in the initial alloy Zn5Al. Anodic curves 2-4 for alloys containing 0.005-0.05% calcium are located to the right of the curves for the original alloy, which indicate the lower anode corrosion rate of these alloys.
Table 1. Electrochemical characteristics of zinc-aluminum Zn5Al alloy, doped with calcium in the ambient of NaCl electrolyte.
|
Ambient |
Content of |
Electrochemical properties |
Corrosion rate |
||||
|
-E. |
E corr . |
–Epa. |
–Erep. |
i corr. ∙ 10 -2 |
K ∙ 10 -3 |
||
|
V |
A/m 2 |
g/m 2∙ h |
|||||
|
0.03% NaCl |
– |
1.050 |
1.060 |
0.915 |
0.930 |
1.02 |
0.34 |
|
0. 005 |
1.040 |
1,005 |
0.860 |
0.905 |
0.39 |
0.13 |
|
|
0. 01 |
1.010 |
1.025 |
0.880 |
0.940 |
0.29 |
0.10 |
|
|
0. 05 |
1.015 |
1,035 |
0.890 |
0.945 |
0.48 |
0.16 |
|
|
0. One |
1.0 30 |
1,035 |
0.895 |
0.940 |
0.51 |
0.17 |
|
|
0. 3 |
1,035 |
1.050 |
0.905 |
0.960 |
0.61 |
0.20 |
|
|
0.3% NaCl |
– |
1.070 |
1.080 |
0.935 |
0.950 |
1.05 |
0.35 |
|
0. 005 |
1.060 |
1.030 |
0.880 |
0.925 |
0.43 |
0.14 |
|
|
0. 01 |
1.030 |
1.050 |
0.900 |
0.960 |
0.34 |
0.11 |
|
|
0. 05 |
1,035 |
1.060 |
0.910 |
0.965 |
0.52 |
0.17 |
|
|
0. One |
1.050 |
1.060 |
0.915 |
0.960 |
0.55 |
0.18 |
|
|
0. 3 |
1,055 |
1.070 |
0.925 |
0.980 |
0.64 |
0.21 |
|
|
3% NaCl |
– |
1.100 |
1.115 |
0.965 |
0.980 |
1.09 |
0.36 |
|
0. 005 |
1.090 |
1.060 |
0.910 |
0.955 |
0.49 |
0.16 |
|
|
0. 01 |
1.060 |
1.080 |
0.930 |
0.990 |
0.38 |
0.13 |
|
|
0. 05 |
1.065 |
1.090 |
0.940 |
0.995 |
0.56 |
0.19 |
|
|
0. One |
1.080 |
1.090 |
0.945 |
0.990 |
0.59 |
0.20 |
|
|
0. 3 |
1.085 |
1.100 |
0.955 |
1.010 |
0.68 |
0.23 |
|
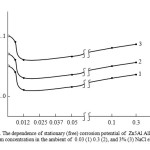 |
Fig1:The dependence of stationary (free) corrosion potential of Zn5Al Alloy on the calcium concentration in the ambient of 0.03 (1) 0.3 (2), and 3% (3) NaCl electrolyte Click here to View Figure |
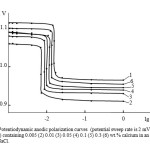 |
Fig2: Potentiodynamic anodic polarization curves (potential sweep rate is 2 mV / s) Zn5Al alloy (1) containing 0.005 (2) 0.01 (3) 0.05 (4) 0.1 (5) 0.3 (6) wt.% calcium in an electrolyte of 3% NaCl.Click here to View Figure |
According to the data in Table 2 and in Fig. 3, anodic behavior of the Zn55Al alloy, doped with calcium show that calcium supplements within 0.005-0.05 wt. % Shift (free) corrosion potential of the source Zn55Al alloy in a positive area. However, further increase in calcium content in the alloys up to 0.3 wt. %, Efree.korr shifts to negative region and this is most noticeable growth of corrosion potentials ( Ekorr. ) , pitting ( Ep.a ) and repassivation ( Erep. ) in the negative direction. The above mentioned features observed in the three studied environments.
Fig. 4 shows the Potentiodynamic anodic polarization curves of Zn55Al alloy, doped with calcium, an electrolyte ambient in 3% aqueous NaCl, showing that the curves 2-4 alloy containing 0.005-0.05% calcium shifted to more positive values of the potential compared to the curve 1 for Zn55Al original alloy, that indicates a lower anode corrosion rate of these alloys.
In general, the studied electrochemical properties of zinc – aluminum Zn5Al and Zn55Al alloys, doped with calcium, become possible to obtain corrosion coatings with optimal calcium concentration of about 0.005-0.05 wt.%. The corrosion rate of these alloys is 2-3 times lower than the parent alloy and they may be used as anticorrosive coatings for corrosion protection of steel structures.
Table 2.Electrochemical characteristics of zinc-aluminum Zn55Al alloy, doped with calcium in NaCl electrolyte ambient.
|
Ambient a |
Content of |
Electrochemical properties |
Corrosion rate |
|||||
|
-E. |
E corr . |
–Epa. |
–Erep. |
i corr. ∙ 10 -2 |
K ∙ 10 -3 |
|||
V |
A/m 2 |
g/m 2∙ h |
||||||
|
0.03% NaCI |
– |
0.970 |
0.990 |
0.850 |
0.870 |
0.30 |
0.101 |
|
|
0.005 |
0.930 |
0.920 |
0.800 |
0.845 |
0.16 |
0.054 |
||
|
0.01 |
0.950 |
0.960 |
0.820 |
0.880 |
0.10 |
0.034 |
||
|
0.05 |
0.955 |
0.970 |
0.830 |
0.885 |
0.12 |
0.040 |
||
|
0.1 |
0.970 |
0.990 |
0.845 |
0.880 |
0.14 |
0.047 |
||
|
0.3 |
0.990 |
1.010 |
0.865 |
0.900 |
0.13 |
0.044 |
||
|
0.3% NaCI |
– |
1.000 |
1.020 |
0.880 |
0.890 |
0.33 |
0.111 |
|
|
0.005 |
0.960 |
0.955 |
0.820 |
0.865 |
0.17 |
0.057 |
||
|
0.01 |
0.980 |
0.990 |
0.840 |
0.900 |
0.11 |
0.037 |
||
|
0.05 |
0.985 |
1.000 |
0.850 |
0.905 |
0.13 |
0.044 |
||
|
0.1 |
1.000 |
1.015 |
0.865 |
0.900 |
0.16 |
0.054 |
||
|
0.3 |
1.020 |
1.030 |
0.890 |
0.920 |
0.15 |
0.050 |
||
|
3% NaCI |
– |
1.020 |
1.040 |
0.900 |
0.920 |
0.37 |
0.124 |
|
|
0.005 |
0.980 |
0.975 |
0.840 |
0.895 |
0.20 |
0.067 |
||
|
0.01 |
1.000 |
1.010 |
0.860 |
0.930 |
0.15 |
0.050 |
||
|
0.05 |
1.005 |
1.020 |
0.870 |
0.935 |
0.18 |
0.060 |
||
|
0.1 |
1.020 |
1.035 |
0.885 |
0.930 |
0.22 |
0.074 |
||
|
0.3 |
1.040 |
1.060 |
0.910 |
0.950 |
0.21 |
0.070 |
||
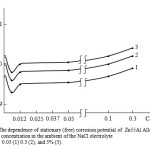 |
Fig3: The dependence of stationary (free) corrosion potential of Zn55Al Alloy on the calcium concentration in the ambient of the NaCl electrolyte 0.03 (1) 0.3 (2), and 3% (3).Click here to View Figure |
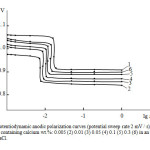 |
Fig4: Potentiodynamic anodic polarization curves (potential sweep rate 2 mV / s) of Zn55Al alloy (1) containing calcium wt.%: 0.005 (2) 0.01 (3) 0.05 (4) 0.1 (5) 0.3 (6) in an electrolyte of 3% NaCl. Click here to View Figure |
Conclusion
Investigating potentiodynamic method indicates that the following patterns of change in the electrochemical characteristics of the Zn5Al and Zn55Al alloys, doped with calcium in the ambient of NaCl electrolyte at 0.03, 0.3 and 3% concentrations indicated that
– free corrosion potential of Zn5Al and Zn55Al alloys doped with calcium has an extreme character, increasing the concentration of chloride ions helps to reduce the values of potential free corrosion
– Increasing concentration of the alloying, pitting and repassivation potentials of element are moved to a positive, and increasing concentration of chloride ions move to the negative region
– Corrosion rate of original alloy is reduced by 2-3 times when the content of calcium down to 0.3%.
References
- Krakowiak, S.; Sobaszek, M. Korozia Elekteromchemia. 2010, 2
- Youssef, Kh.M.S.; Koch, C.C.; Fedkiw, P.S. Corrosion Sci., 2004, 52, 82
- Yamashita, M.; Inagaki, J.; Yoshida, K.; Yamaji, T; Ishikawa, H.; Okuma, T. NKK Technical review, 2002, 87, 7
- Amini Najafabadi, R.; Meysami, A. H.; Sharifi, H.,”Korrosia aluminiviq slpavov” LAP LAMBERT Academic Publishing, Germany, 2013, 77
- Sinyavsky, V.S.; Volkov, V.D.; Kalinin, V.D., Metallurgy, Moscow, 1979, 640
- Shiyan, Z.; Qing L.; Xiaokui Y.;, Xiankang Z.;, Yan D.; , Fei L.; Materials Characterization, 2010, 61, 270
- Freiman L.I.; Makarov V.A.; Bryksin, I.E. Ed. Acad. Kolotyrkin Y.M. Khimiya, 1972, 240
- Umarov, T.M.; Ganiev, I.N., Donish Dushanbe, 2007, 258
- Kolotyrkin, J.M. “Metal e korrozia(Metal and Corrosion)”, Moscow: Metallurgia, 1985, 88
- Umarova, T.M.; Ganiev, I.N. , Donish Dushanbe, 2009, 232

This work is licensed under a Creative Commons Attribution 4.0 International License.









