2-{2"-Carbomyl-5"-[3'-Amino-2'-Methylmono/Dihalosubstituted Quinazolin-4'(3'h)-Onomethylene]-1",3",4"-Oxadiazol-2"-Yl}-4,5-Dihydroimidazolines as Potential Antihypertensive Agents
Mirdula Tyagi
Medicinal Chemistry Lab, Department of Chemistry Meerut College, Meerut 250004, U.P. (India)
DOI : http://dx.doi.org/10.13005/ojc/300243
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2014
'Twelve new 2- {2"-carbomyl - 5" - [3'-amino-2' – methylmono / dihalosubstituted quinazolin- 4' (3'H) - onomethylene] – 1",3",4" - oxadiazol-2"-yl} -4, 5- dihydroimidazolines were prepared and evaluated for their cardiovascular activity. The most active compound of this series is 2-{2"-carbomyl-5"-[3'-amino-2'-methyl-6-bromoquinazolin-4'(3'H)-onomethylene]-",3",4"-oxadiazol-2"-yl}-4,5-dihydroimidazolines i.e. compound VIc.
KEYWORDS:Quinazolinonyl oxadiazoles; acute toxicity studies; hypotensive activity; dogs; synthesis Quinazolinonyloxadiazolyl-imidazolines; acute toxicity studies; hypotensive activity dogs; synthesis.
Download this article as:| Copy the following to cite this article: Tyagi M. 2-{2"-Carbomyl-5"-[3'-Amino-2'-Methylmono/Dihalosubstituted Quinazolin-4'(3'h)-Onomethylene]-1",3",4"-Oxadiazol-2"-Yl}-4,5-Dihydroimidazolines as Potential Antihypertensive Agents. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Tyagi M. 2-{2"-Carbomyl-5"-[3'-Amino-2'-Methylmono/Dihalosubstituted Quinazolin-4'(3'h)-Onomethylene]-1",3",4"-Oxadiazol-2"-Yl}-4,5-Dihydroimidazolines as Potential Antihypertensive Agents. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3592 |
INTRODUCTION
Research in the field of imidazoline derivatives has yielded a number of clinically useful anti-hypertensive drugs. Imidazoline derivatives of different heterocyclic nucleus have shown potent pharmacological properties like antiinflammatory1. analgesic2, CNS-depressant3, anticonvulsant4 and hypotensive5-6. Importantly, substitution at 2-position of imidazoline nucleus plays pivotal role in molecular designing of some cardiovascular agents like clonidine, which lowered the blood pressure. Furthermore, substitution at 2-position of imidazoline nucleus by different heterocyclic moieties, plays a pivotal role in delineating the cardiovascular activity7-8. Moreover, quinazolinones9-10 and oxadiazoles11-12 have also been reported to possess potent antihypotensive activity. With an aim to develop better hypotensive agents, it was thought worthwhile to synthesize a new series of 2-substituted imidazoline derivatives bearing oxadiazolyl and quinazolinonyl moieties. All the newly synthesized compounds (synthetic route is given in scheme) were evaluated for their cardiovascular activity and acute toxicity studies. Moreover structures of all the compounds were delineated by spectral analysis.
MATERIALS AND METHODS (EXPERIMENTAL)
Chemistry
All melting points are uncorrected. The purity of the compounds were checked by TLC on silica gel-G plates and spots were located by iodine. IR spectra were recorded on Beckman-Acculab-10-spectrophotometer (umax in cm-1) 1H-NMR spectra was recorded on Bruker-400-FT instrument. Mass spectra were recorded on Jeol- JMS D-300 spectrophotometer.
Synthesis
The required 3-amino – 2- methylmono / dihalosubstitutedquinazolin – 4 (3H) – ones la-Id, were synthesized according to the reported method13, which on reaction with ethylchloroacetate, in the presence of anhydrous K2Co3 gives ethyl-3-amino-2-methylmono/dihalosubstituted-quinazolin-4(3H)-onoacetates (Ila-IId). Compound Ila-IId on treatment with semicarbazide in methanol gave l-[3′-amino-2′- methylmono / dihalosubstitutedquinazolin -4′ (3’H) – onoacetyl]- semicarbazides (IIIa-IIId). These compounds were reacts with conc. H2SO4 and then neutrallized with liquid ammonia to yield cyclized products i.e. 2-amino-5- [3′-amino-2′- methyl-mono / dihalosubstitedquinazolin – 4′ (3’H)-onomethylene] – l,3,4- oxadiazoles IVa-IVd.2 – Aminochloroacetyl-5- [3′-amino – 2- methylmono/ dihalosubstitutedquinazolin-4′ (3’H)-onomethylene]-l,3,4-oxadiazoles i.e. Va-Vd were prepared by the addition of chloroacetylchloride drop by drop into the solution of compound IVa-IVd, these compounds were cyclized to imidazolines Vla-VId, by the addition of ethylenediamine and sulphur.
Synthesis of ethyl-3- amino- 2- methyImono/ dihalosubstituted- quinazoIin – 4 (3H) – onoacetates (Ila-IId)
A mixture of 3-Amino-2-methyl-mono / dihalosubstitutedquinazolin-4 (3H)-one (0.01 mole), ethylchloroacetate (0.01 mole) and anhydrous K2CO3 (5g) in dry acetone (80 ml) were refluxed for 15 hours on a steam bath. The excess of the solvents were distilled off and the resulting solid mass poured into ice cold water, filtered and recrystallised from appropriate solvents. Physical and analytical data of compounds are given in table I.
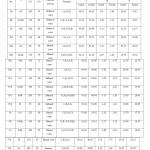 |
Table-1 – Physical properties of compounds (la-Id), (lla-lld), (Illa-llld), (IVa-lVd), (Va-Vd) and (Vl-VId) Click here to View table |
Compound IIa: IR (cm-1; selected lines); 3250 (NH), 3060 (C-H aromatic), 2918 (methyl C-H stretch), 2845 (CH2), 1740 (C=O), 450 (C-I stretch). 1H-NMR (CDC13): d 9.60 (ss, 1H, NHCH2), 7.25-7.80 (m, 3H, Ar-H), 4.40 (d, 2H, CH2NH), 4.10 (q, 2H, J=7Hz, COOCH2CH3), 2.48 (s, 3H, CH3), 1.22 (t, 3H, J=7Hz, COOCH2CH3) (ppm).  
[MS]:[M]+m/z 387.
Synthesis of l-[3′- amino-2′- methylmono/dihalosubstituted- quinazoIin-4-(3’H)-onoacetyl]-semicarbazides (IIIa-HId)
A mixture of 3-(ethylacetylamino)- 2- methyl- mono /dihalosubsti tutedquina zolin-4 (3H)- ones (0.075 mole) and semicarbazide (0.075 mole) in methonal (70ml) were refluxed on a steam bath for about 8 hr. The excess of the solvents were distilled off and the viscous mass poured into ice cold water, filtered and recrystallised from appropriate solvents. Physical and analytical data of compounds IIIa-IIId are given in table I.
Compound IIIa: IR (cm-1, selected lines); 3200 (NH), 3050 (aromatic C-H), 2918 (methyl C-H stretch), 2853 (CH2), 1700 (C=O), 1600 (C=N), 1580 (C….C of aromatic ring), 500 (C-I stretch). 1H-NMR (CDC13): d 9.55 (ss, 1H, NHCH2), 8.80 (m, 4H, NHNHCONH2), 7.52-6.80 (m, 3H, Ar-H), 4.30 (d, 2H, NHCH2), 2.52 (s, 3H, CH3) (ppm). MS: [M]+m/z416.
Synthesis of 2-amino-5- [3′-amino-2′- methylmono/dihalo- sub- stitutedquinazolin- 4′(3’H)- onomethylene] 1,3,4- oxadiazoles (IVa-IVd).
Conc. H2SO4 (15ml) and a mixture of l-[3′-amino -2′- methyl – mono/ dihalosubstitutedquinazolin- 4 (3H) – oneacetyl] semicarbazides (0.05 mole) were kept overnight at room temperature, poured into ice cold water, neutrallised with liquid NH3 and filtered. The product obtained were recrystallised from appropriate solvents. Physical and analytical data of compounds IVa-IVd are given in table-I.
Compound IVa: IR (cm-1, selected lines; 3355 (NH2), 3053 (aromatic C-H), 2920 (methyl C-H stretch), 2845 (CH2), 1790 (CO), 1600 (C=N), 1580 (C….C
of aromatic ring), 1080 (C-O-C). 1H-NMR (CDC13): d 9.40 (ss, 1H, NHCH2), 8.20 (s, 2H, NH2), 7.58-7.70 (m, 3H, Ar-H), 4.30 (d, 2H, NHCH2), 2.70 (s, 3H, CH3) (ppm).
MS: [M]+ m/z 398
Synthesis of 2- [aminochloroacetyI- 5- (3′-amino-2′- methyI-
mono/dihalosubstitutedquinazolin-4(3’H)-onomethylene]-l,3,4-oxadiazoles Va-Vb.
To a well stirred solution of compounds IVa-IVd (0.01 mole) in dry chloroform (40 ml) chloroacetylchloride (0.02 mole) was added at 0°C dropwise during 1 hr. The reaction mixtures were stirred for 5 hr more cooled and poured into ice water. The resulting mixtures were filtered and recrystallised from appropriate solvents. Physical and analytical data of compounds Va-Vd are mentioned in table-I
Compound Va: IR (cm-1, selected lines); 3045 (aromatic C-H), 2870 (CH2), 1770 (C=0), 1635 (C=N), 1550 (C….C of aromatic ring), 1010 (C-O-C), 690 (C-C1). 1H-NMR (CDC13): d 9.40 (ss, IK, NHCH2), 8.49 (hump, 1H, NHCO), 7.10-8.20 (m, 3H, Ar-H), 4.72 (s, 2H, CH2C1), 4.35 (d, 2H, NHCH2), 2.50 (s, 3H, CH3) (ppm).
MS: [M]+m/z474.
Synthesis of 2-{2″-carbomyl-5″-[3′-amino-2′-methylmono/ dihalosubstitutedquinazolin-
4′(3’H)-onomethylene]-l”,3″,4″-oxadiazol -2″-yI}-4,5-dihydroimidazolines Vla-VId.
To a mixture of compounds Va-Vd (0.01 mole) in toluene (dry 100 ml) and sulphur (0.02 mole), ethylenediamine (0.01 mole) was added dropwise at 110°C during Ihr. The reaction mixtures were refluxed for 4 hrs. till the evolution of hydrogen sulphide ceased. It is filtered hot and the filterate concentrated and poured into crushed ice. The resulting solids were recrystallised from appropriate solvents. Physical and analytical data of compounds Vla-VId are given in table-I
Compound VIa: IR (cm-1, selected lines); 3240 (NH), 3040 (aromatic C-H), 2930 (methyl C-H stretch), 2850 (CH2), 1700 (C=O of NHCO), 1620 (C=N), 1510 (C…..C of aromatic ring), 1040 (C-O-C). 1H-NMR (CDC13): 5 9.55 (ss, 1H, NHCH2), 8.40 (bs, 1H, NHCO), 7.45-8.10 (m, 3H, Ar-H), 5.65 (bs, 1H, NH of imidazoline ring), 4.30 (d, 2H, NHCH2), 3.80-3.58 (m, 4H, CH2-CH2 of 4,5-dihydroimidazoline ring), 2.48 (s, 3H, CH3) (ppm).
MS: [M]+m/z 494.
BIOLOGICAL ACTIVITIES
The present study was carried out on adult normotensive mongrel dogs (10-20 kg) or on cats (3-4 kg) and charles foster albino mice (18-25gm). The dogs/cats were divided into two groups of 5 animal each. One of the groups was treated as control group while another group was treated as test group. Dogs/cats were anaesthetized with α-chloralose (80 mg/kg i.p.) injected intravenously and maintained on positive pressure artificial respiration by cannulation of the trachea in order to avoid reflex change in respiration. The right femoral vein was cannulated in each case with on indevelling polyethene tube. The blood pressure was recorded either from the left common carotid artery by means of a mercury manometer on smoked Kymography paper or from femoral artery on one channel of “Encardiorite” (India) polygraph using stathus P23 transducer. Electrocardiogram (Lead II) was recorded on one channel of “Encardiorite” (India) polygraph in some of the experiments. The heart rate was calculated from the pressure pulse tracing in all the experiments. The newly synthesized compounds (test drugs) were administered intravenously through an indevelling polyethylene cannula by dissolving them in propylene glycol and the effect on blood pressure (B.P.), heart rate (H.R.) and pressor responses evoked either by carotid occlusion (CO) or intravenous noradrenaline (NA) 1-2 mg/kg injection was studied. 0.25 ml of propyleneglycol was injected as vehicle to see the effect of vehicle on the parameters in the control group of animals. Injection of 0.25ml of propylene glycol induced a mild and transient decrease of 5 mmHg in blood pressure without affecting the CO and NA responses. The toxicity study was carried out on mice of either sex. Approximate 50% lethal dose (ALDso) of the all the compounds was determined in albino mice. The mice of either sex weighing between 18-25 gm were used for the study. The drugs were injected by intraperitonial (i.p.) route at different dose levels in separate groups of animals. From the data obtained ALD5o was calculated according to the method14. The results were analysed by using student’s t-test.
Toxicity Studies: ALD50
Approximate ALD50 values of all the compounds were determined by observing 50% mortality after 24 hours.
RESULTS
Hypotensive activity was determined according to the reported method15. Cardiovascular activities of these compounds are presented in table (2, 3, & 4). All the four 2-amino-5- (3′-amino-2′- memylmono/ dihalosubstitutedquinazolin-4′ (3’H)- onomethylene]- l,3,4-oxadiazoles (IVa-IVd; table 2) exhibited mild hypotensive activity of varying degree (10-30 mmHg) and duration (10-22 minutes) without affecting the carotid occlusion (CO) and noradrenaline (NA) pressor responses. Compound Ic i.e. 2-amino-5-(3′-amino-2′-methyl-6′-bromo-quinazolin-4′(3’H)-onomethylene]-l,3,4-oxadiazole showed an immediate fall in blood pressure of l0mmHg followed by a delayed fall of 30 mmHg. The hypotensive activity of this compound lasted for 22 minutes. As these compounds IVa-IVd did not affect the CO and NA responses and had very short duration of action, therefore, they appear to be acting on the smooth muscles of blood vessels (direct vasodilators). Compounds (Va-Vd; table 3) of stage II exhibited better cardiovascular activity than their parent compounds (IVa-IVd; table-2). These compounds exhibited hypotensive activity of varying degree (20-70 mmHg) of duration (20-30 minutes) without affecting CO and NA responses and heart rate. Compound Vc showed an immediate fall in blood pressure 70 mmHg followed by a delayed fall of 30mmHg. The hypotensive activity of this compound lasted for 20 minutes without affecting the pressor responses (CO and NA) and heart rate (HR). Further, the cyclization of these above2-aminochloroacetyl-5-(2-methylmono/dihalosubstituted-quinazolin-4(3H)-onomethylene)-l,3,4-oxadiazoles into their corresponding carbomyl (CONH) imidazolines i.e. 5-membered ring structure (Vla-VId; table-4) exhibited potent cardiovascular activity of varying degree (10-60 mmHg) of duration (30-50 minutes). All these compounds Vla-VId showed more’ potent cardiovascular activity than their parent compounds (Va-Vd; table-3) due to the presence of imidazoline ring. Compound VIc i.e. when quinazolinone was substituted with Br at 6-position, showed an immediate fall in blood pressure (60 mmHg), followed by gradual fall in blood pressure (40 mmHg) at a dose of 2.5 mg/kg i.v. The hypotensive activity of this compound lasted for about 50 minutes. As this compound (compound VIc) exhibited the promising activity at a dose of 2.5 mg/kg i.v., it was therefore thought worthwhile to study this compound at three graded doses (1.25, 2.5 and 5.0 mg/kg i.v.). Interestingly this compound was associated with either inhibition or blockade of CO without affecting the NA response, which might be suggestive of central site of action of this compound. However, compound VIc has shown tachycardia (increase in heart rate) 1-2 beats per minutes, 2-3 beats per minutes and 3-4 beats per minutes at a dose of 1.25,2.5 and 5.0mg/kg i.v. respectively. The results of the cardiovascular activity are mentioned in table (4). Compound VId has also exhibited a potent hypotensive fall of 50 mmHg. The hypotensive activity of this compound lasted for 30 minutes with inhibition of both CO and NA. Such cardiovascular profile is suggestive of peripheral site of action of this compound. Compound VIa exhibited an immediate fall of blood pressure 40 mmHg followed by a delayed fall of 30mmHg. The hypotensive activity of compound VIa was lasted for 35 minutes with potentiation of CO without affecting the NA response, which might be suggestive of central site of action of this compound.
![Table-2 Cardiovascular activity of 2-amino-5-[3'-amino- 2'- methylmono/ dihalosubstituted quinazolin-4'(3lH)-onomethylene]-1,3,4 - oxadiazoles.](http://www.orientjchem.org/wp-content/uploads/2014/06/Vol30_No2_Synth_Mirdu_t1-150x150.jpg) |
Table 2:Cardiovascular activity of 2-amino-5-[3′-amino- 2′- methylmono/ dihalosubstituted quinazolin-4′(3lH)-onomethylene]-1,3,4 – oxadiazoles. |
![Table-3 Cardiovascular activity of 2— (aminiochloroacety) -5- [31amino-21 -methyl-mono/ dihalosubstitutedquinazolin- 41 (31H)) - onomethylene] - 1,3,4 -oxadiazoles.](http://www.orientjchem.org/wp-content/uploads/2014/06/Vol30_No2_Synth_Mirdu_t3-150x150.jpg) |
Table 3: Cardiovascular activity of 2— (aminiochloroacety) -5- [31amino-21 -methyl-mono/ dihalosubstitutedquinazolin- 41 (31H))-onomethylene] – 1,3,4 -oxadiazoles. |
![Table-4 Cardiovascular activity of 2-{2"-carbomyl-5"- [3'-amino-2'- methylmono/ dihalosubstitutedquinazolin- 4'(3lH)- onomethylene]-1",3",4"-oxadiazol- 2"-yl}-4,5- dihydroimidazolines.](http://www.orientjchem.org/wp-content/uploads/2014/06/Vol30_No2_Synth_Mirdu_t4-150x150.jpg) |
Table 4: Cardiovascular activity of 2-{2″-carbomyl-5″- [3′-amino-2′- methylmono/ dihalosubstitutedquinazolin- 4′(3lH)- onomethylene]-1″,3″,4″-oxadiazol- 2″-yl}-4,5- dihydroimidazolines. |
DISCUSSION
It is interesting to point out that compounds of third stage have shown different pharmacological profile (clonidine like centrally acting as compound VIa, VIb & VIc, secondly a purely peripheral adrenergic blocking type as compound VId. Furthermore, all the compounds i.e. Vla-VId of stage third exhibited the more potent antihypertensive activity than their parent compounds (IVa-IVd and Va-Vd). On the contrary, all the compounds of stage first (IVa-IVd) and stage second (Va-Vd) did not affect the CO and NA responses and had short duration of action. They appear to be acting directly on the smooth muscles of the blood vessels (direct vasodilators). Moreover, it is also evident from the results that presence of imidazoline ring with oxadiazolyl and quinazolinonyl moieties is beneficial for cardiovascular activity. Moreover, the cyclisation of compound Va-Vd to Vla-VId i.e. imidazolines increases the cardiovascular activity in terms of magnitude as well as duration.
ACKNOWLEDGEMENTS
We are thankful to the CDRI, Lucknow for the elemental and spectral analysis. The contribution of Dr. Atul Kumar and Dr. P.M.S. Chauhan, Scientist, Medicinal Chemistry Division, CDRI, Lucknow is also gratefully acknowledged.
REFERENCES
- Ueda Y and Sato K: 2-Aminoimidazoline derivatives, Chem. Abstr. 1986,104 (21), 186413f.
- Franzmair R. and Enzenhofer R: Arylimidazoline derivatives, Chem. Abstr. 1980,92(7), 58779b.
- Bigg D.M., Morel C.M. and Sevrin M: Imidazoline derivatives and their therapeutic use, Chem. Abstr. 1985,103 (9), 71317q.
- Hetzheim A., Weiher B. and Morgenstorn E: l-(Acylamino)-2- (phenylethylamino)-4-phenylimidazoles as antihypertensive agent, Chem. Abstr. 1992,117 (23), 234014u.
- Kamitani T., Katamoto M., Tsujioka K., Terai T., Ohtsuka M., Ono T., Kikuchi H. and Kumada S: The hypotensive effect of 2-(5-chloro-5-phenoxyanilino)-2-imidazolines, J. Pharmacol 1985, 39 (2), 251-261.
- Carron C.L.C., Bucher B.P. and Jullien A.F: 2-Amino-2-imidazoline derivatives, Chem. Abstr. 1974, 80 (3), 14927w.
- Cairns D. and Sneader W: Synthesis of Novel benzylimidazolines possessing antihypertensive and sedative activity, Arch. Pharm. 1989, 322 (7), 391-393.
- Kluge M., Whiting R.L. and Chritie G.A: Benzodioxane-imidazoline compounds and their use, Chem. Abstr. 1983, 99(7), 53769H
- Botros S., Saad S.F: Synthesis, antihypertensive and D-adrenoreceptor antagonist activities, Eur. J. Med. Chem. 1989, 24 (6), 585-590.
- Beveung W.N., Pertyka R.A: Optionally substituted 1,2,3,5-tetrahydroimidazo [2,1-b] quinazolin-2-ones, Chem. Abstr. 1977, 86 (11), 72688d.
- Kumar A., Jaju B.P., Gurtu S., Sinha J.N. and Shanker K: 5-Substituted-2-{4-[4-(m-chlorophenyl)-piperazin-1-yl]-7-(trifluoro-methyl)-quinazolin-3-yl} -1,3,4-oxadiazoles as hypotensive agents, Ind. J. Chem. 1988, 27(1), 93-95.
- Ramalingam T., Deshmukh A.A., Sattur P.B., Seth U.K. and Naik S.R: Synthesis and pharmacology of 2,6-disubstituted-1,3,4-oxadiazoles, J. Ind. Chem. Soc. 1981, 58 (3), 269-271.
- Kumar A., Singh S., Saxena A.K. and Shanker K: Synthesis of quinazolidinylpyrimidinediones and their antiinflammatory activity, 1988, 27 (5), 443-447.
- Smith Q.E: Pharmacological Screening tests progress in Medicinal Chemistry, Butterworths, London 1960,1.
- Kumar A., Gurtu S., Sinha J.N., Bhargava K.P. and Shanker K: Synthesis and hypotensive activity of trisubstituted quinazolinones, 1985, 20 (1), 95-96.

This work is licensed under a Creative Commons Attribution 4.0 International License.









