Synthesis and Characterization of 3-(1-Hydroxy Naphthalene-2-yl)-5-(Furan-2-yl)-1-Substituted Pyrazolines
S.E.Bhandarkar
Department of chemistry, Govt. Vidarbha Institute of Science and Humanities, Amravati (MS) India
DOI : http://dx.doi.org/10.13005/ojc/300150
Article Received on :
Article Accepted on :
Article Published : 04 Mar 2014
2-acetyl-1-naphthol 2 is prepared by Modified Nenchi’s method which on treatment with furfuraldehyde and KOH gives 1-(1-hydroxy naphthalen-2-yl)-3-(furan-2-yl) prop-2-ene-1-ones 3 in excellent yield. The chalcone 3 when subjected to hydrazine / phenyl hydrazine/ semicarbazide / 2,4 dinitro phenyl hydrazine / isonicotinic acid hydrazide in DMF solvent gives 3-(1-hydroxy naphthalene-2-yl)-5-(furan-2-yl)-1-substituted pyrazolines 4, 5, 6, 7 and 8 in 35-45% yield. The structural assignments to the compounds 4, 5, 6, 7 and 8 are based on their elemental analysis and spectral data.
KEYWORDS:Synthesis; Characterization; Pyrazolines.
Download this article as:| Copy the following to cite this article: Bhandarkar S. E. Synthesis and Characterization of 3-(1-Hydroxy Naphthalene-2-yl)-5-(Furan-2-yl)-1- Substituted Pyrazolines. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Bhandarkar S. E. Synthesis and Characterization of 3-(1-Hydroxy Naphthalene-2-yl)-5-(Furan-2-yl)-1- Substituted Pyrazolines. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2292 |
Introduction
The literature survey reveals that pyrazoline derivatives have been studied extensively because of their ready accessibility, diverse chemical reactivity, broad spectrum of biological activity6-8and variety of Industrial applications.9,10 Pyrazolines with sulphonamidoaryl substituent at 3-position show cerebroprotective11, antidepressant activity12 , anti-implantation activity13, hypoglycemic activity14. Due to this vital biological roll of pyrazoline derivatives, it was thought of interest to synthesize titled pyrazoline derivatives . Thus we present herein the synthesis of the titled compounds. It has been observed that substituted flavanones are the best starting compounds for the preparation of 4-aroyl derivatives of pyrazoline. Present work deals with the synthesis of some new pyrazolines and their characterization by spectral analysis ( IR, 1H NMR )
EXPERIMENTAL
All the melting points were taken in silicon oil bath with open capillary tubes and are uncorrected. 1 H NMR spectra were recorded on a Brucker AC300 FNMR spectrometer(300MHz), using TMS as an internal standard. IR spectra were recorded on a Nicolet-Impact 400 FT-IR spectrometer. Thin Layer Chromatography on silica gel-G, was used to check the purity of the compounds. Microanalysis of nitrogen was obtained on Colman 29-N analyzer.
Experimental Section
Procedure for the synthesis of 2-acetyl-1-naphthol 2
In hot glacial acetic acid (80ml), fused ZnCl2(50 gm) was added and refluxed till dissolved, then powdered 1-naphthol (30gm )was added and the mixture was refluxed for about 8 hours then cooled & poured in acidulated water. The solid obtained was filtered, washed, dried and recrystallized from rectified spirit to obtain compound 2. Physical data of the compound is given in table 1
2: IR (KBr) : 1650 (C=O), 3412 (-OH) NMR (CDCl3 + DMSO-d6) : δ 2.35 (S,3H,CH3), δ 7.11-6.88 (m, 6H, Ar-H), δ 9.83 (S,1H,-OH)
Synthesis of 1-(1-hydroxy naphthalen-2-yl)-3-(furan-2-yl) prop-2-ene-1-one (3):
2-acetyl-1-naphthol ( 0.01mole ) and furfuraldehyde (0.02 mole) were added in ethanol solvent (20ml). To this mixture KOH (10%, 10ml) solution was added dropwise with constant stirring. The reaction mixture was kept overnight. Then the mixture was poured over crushed ice & little HCl. The product was filtered and recrystallized from ethanol to obtain the compounds (3). The physical data is given in Table 1
3: IR (KBr):1650 (C=O), 3412(-OH), 1155(C-O-C) NMR (CDCl3 + DMSO-d6) : δ 6.98-7.46 (m,9H, Ar-H), δ 8.057 (d,1H =CH) , δ 8.099(d,1H =CH)
δ 9.63 (S,1H,-OH)
Synthesis of 3 – ( 1 – hydroxy naphthalen – 2 – yl ) – 5 – ( furan-2-yl ) – 1-substituted pyrazolines(4-8):
1-(1-hydroxy naphthalen-2-yl)-3-(furan-2-yl) prop-2-ene-1-ones (0.01mole) & hydrazine / phenyl hydrazine/ semicarbazide / 2,4 dinitro phenyl hydrazine / isonicotinic acid hydrazide (0.01 mole) were added to DMF (20 ml ) and refluxed for 2 hours . The cooled reaction mixture was diluted with water & the semisolid so obtained was triturated with ethanol to get a solid which was recrystallised from ethanol–acetic acid mixture to get titled pyrazolines in 45-51% yield . The physical data is given in Table 1
Table 1: Physical and analytical characterization data of newly synthesized compounds
| Sr.No. | Compound No | Molecular formula | R | Melting Point 0C | %Yield | % Nitrogen | R.F.Value | |
| Found | Calculated | |||||||
| 1 | 2 | C12H10O2 | — | 980C | 72% | — | — | |
| 2 | 3 | C17H12O3 | — | 1260C | 71% | — | — | |
| 3 | 4 | C17H14N2O2 | H | 1100C | 38% | 10.00 | 10.07 | 0.79 |
| 4 | 5 | C23H18N2O2 | C6H5 | 1000C | 40% | 7.81 | 7.91 | 0.88 |
| 5 | 6 | C18H15N3O3 | CONH2 | 1400C | 45% | 12.99 | 13.08 | 0.87 |
| 6 | 7 | C23H16N4O6 | C6H3N2O4 | 1200C | 40% | 12.56 | 12.61 | 0.78 |
| 7 | 8 | C23H17N3O3 | C5H4NCO | 1170C | 35% | 10.91 | 10.97 | 0.84 |
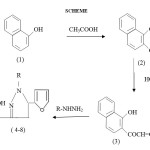 |
Scheme1: Click here to View Scheme |
R = H, C6H5, CONH2, C6H3N2O4, C5H4NCO IR, (KBr):3000 (N-H),
3402(-OH), 1151(C-O-C) NMR (CDCl3 + DMSO-d6) : δ 6.90-7.80 (m,6H, Ar-H), δ 8.051 (d,1H =CH) , δ 8.091(d,1H =CH) δ 9.62 (S,1H,-OH)
IR (KBr):1600 (C=C), 3410(-OH), 1148(C-O-C) NMR (CDCl3 + DMSO-d6) :δ 6.89-7.90 (m,11H, Ar-H), δ 8.049 (d,1H =CH) , δ 8.081(d,1H =CH) δ 9.53 (S,1H,-OH).
IR (KBr):1723 (C=O), 3405(-OH), 1160(C-O-C) NMR (CDCl3 + DMSO-d6) : δ 6.81-7.40 (m,6H, Ar-H), δ 8.041 (d,1H =CH) , δ 8.079(d,1H =CH) δ 9.49 (S,1H,-OH), 6.6 (S,2H,NH2).
IR(KBr):1530,1550(NO2),3412(OH),1155(C-O-C) NMR (CDCl3 + DMSO-d6) : δ 6.81-7.60 (m,9H, Ar-H), δ 8.045 (d,1H =CH) , δ 8.081(d,1H =CH) δ 9.43 (S,1H,-OH).
IR (KBr):1611 (C=O), 3477(-OH), 1170(C-O-C) NMR(CDCl3 + DMSO-d6) : δ 6.95-7.81 (m,10H, Ar-H), δ 8.043 (d,1H =CH) , δ 8.083(d,1H =CH) δ 9.50 (S,1H,-OH).
ACKNOWLEDGEMENT
The author is thankful to the Director, Govt. Vidarbha Institute of Science and Humanities, Amravati.
REFERENCES
- F. Kally, G. Jonzso and L. Kuezoo, Tetrahedron, 21, 5128(1965)
- A.E.A. Sammar and Mhd. Elkasaby, J. Chem. Un. Arab Ribad, 12(1969)
- M.M.Chincholkar and V.S. Jamode, Indian J. Chem.,17B, 623(1979)
- K.T.Borkhade and M.G. Marathey, Indian J. Chem., 10, 48(1972)
- A. Schonberg and M.M. Siddey, J. Am. Chem. Soc., 75, 48(1972)
- M.S.R. Murthy, E.V. Rao and P. Ranganathan, Indian Drugs, 22,1(1985)
- C.W. Noell and C.C. Cheng, J. Med. Chem., 12,545(19690
- M.S.R. Murthy, D.V. Rao and E.V. Rao J. Pharma Science, 45,131(1983)
- G.V. Subbaraju, Ranaga Nayukulu and D. Parameswara Indian J. Heterocycl. Chem., 4,87(1994)
- K.S.Rao and G.V.Subbaraju, Indian J. Heterocycl. Chem., 4,19 (1994)
- V. S. Jamode, H. S. Chandak, Asian J. Chem.15 (2), 2003,897-900.
- G. E. H. Elgemeie, A. M. E. Attia, D. S. Farag, S. M. Sherif, J. Chem. Soc. Perkin Trans., 1, (1994), 1285.
- Erhan Palaska, Mutlu Aytemir, Tayfun Uzbay and Dilek erol, Euro, J. Med., Chem., 36 (2001), 539.
- Batulin Y. U. M. Chem. Abstr., 70, (1969), 2236a.

This work is licensed under a Creative Commons Attribution 4.0 International License.









